CD8+ T cells recognizing a neuron-restricted antigen injure axons in a model of multiple sclerosis
- PMID: 37676734
- PMCID: PMC10617772
- DOI: 10.1172/JCI162788
CD8+ T cells recognizing a neuron-restricted antigen injure axons in a model of multiple sclerosis
Abstract
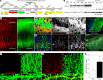
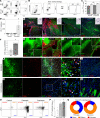
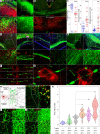
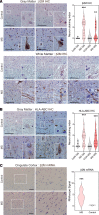
CD8+ T cells outnumber CD4+ cells in multiple sclerosis (MS) lesions associated with disease progression, but the pathogenic role and antigenic targets of these clonally expanded effectors are unknown. Based on evidence that demyelination is necessary but not sufficient for disease progression in MS, we previously hypothesized that CNS-infiltrating CD8+ T cells specific for neuronal antigens directly drive the axonal and neuronal injury that leads to cumulative neurologic disability in patients with MS. We now show that demyelination induced expression of MHC class I on neurons and axons and resulted in presentation of a neuron-specific neoantigen (synapsin promoter-driven chicken ovalbumin) to antigen-specific CD8+ T cells (anti-ovalbumin OT-I TCR-transgenic T cells). These neuroantigen-specific effectors surveilled the CNS in the absence of demyelination but were not retained. However, upon induction of demyelination via cuprizone intoxication, neuroantigen-specific CD8+ T cells proliferated, accumulated in the CNS, and damaged neoantigen-expressing neurons and axons. We further report elevated neuronal expression of MHC class I and β2-microglobulin transcripts and protein in gray matter and white matter tracts in tissue from patients with MS. These findings support a pathogenic role for autoreactive anti-axonal and anti-neuronal CD8+ T cells in MS progression.
Keywords: Immunology; Multiple sclerosis; Neurological disorders; Neuroscience; T cells.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

