Mitapivat reprograms the RBC metabolome and improves anemia in a mouse model of hereditary spherocytosis
- PMID: 37676741
- PMCID: PMC10619498
- DOI: 10.1172/jci.insight.172656
Mitapivat reprograms the RBC metabolome and improves anemia in a mouse model of hereditary spherocytosis
Abstract
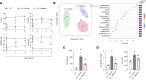
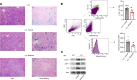
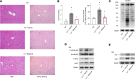
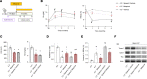
Hereditary spherocytosis (HS) is the most common, nonimmune, hereditary, chronic hemolytic anemia after hemoglobinopathies. The genetic defects in membrane function causing HS lead to perturbation of the RBC metabolome, with altered glycolysis. In mice genetically lacking protein 4.2 (4.2-/-; Epb42), a murine model of HS, we showed increased expression of pyruvate kinase (PK) isoforms in whole and fractioned RBCs in conjunction with abnormalities in the glycolytic pathway and in the glutathione (GSH) system. Mitapivat, a PK activator, metabolically reprogrammed 4.2-/- mouse RBCs with amelioration of glycolysis and the GSH cycle. This resulted in improved osmotic fragility, reduced phosphatidylserine positivity, amelioration of RBC cation content, reduction of Na/K/Cl cotransport and Na/H-exchange overactivation, and decrease in erythroid vesicles release in vitro. Mitapivat treatment significantly decreased erythrophagocytosis and beneficially affected iron homeostasis. In mild-to-moderate HS, the beneficial effect of splenectomy is still controversial. Here, we showed that splenectomy improves anemia in 4.2-/- mice and that mitapivat is noninferior to splenectomy. An additional benefit of mitapivat treatment was lower expression of markers of inflammatory vasculopathy in 4.2-/- mice with or without splenectomy, indicating a multisystemic action of mitapivat. These findings support the notion that mitapivat treatment should be considered for symptomatic HS.
Keywords: Genetic diseases; Hematology; Mouse models; Therapeutics.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

