Health Care Provider Knowledge and Attitudes Regarding Adult Pneumococcal Conjugate Vaccine Recommendations - United States, September 28-October 10, 2022
- PMID: 37676840
- PMCID: PMC10495187
- DOI: 10.15585/mmwr.mm7236a2
Health Care Provider Knowledge and Attitudes Regarding Adult Pneumococcal Conjugate Vaccine Recommendations - United States, September 28-October 10, 2022
Abstract
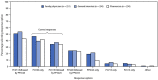
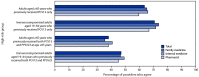
Despite the availability of effective vaccines against pneumococcal disease, pneumococcus is a common bacterial cause of pneumonia, causing approximately 100,000 hospitalizations among U.S. adults per year. In addition, approximately 30,000 invasive pneumococcal disease (IPD) cases and 3,000 IPD deaths occur among U.S. adults each year. Previous health care provider surveys identified gaps in provider knowledge about and understanding of the adult pneumococcal vaccine recommendations, and pneumococcal vaccine coverage remains suboptimal. To assess the feasibility and acceptability domains of the Advisory Committee on Immunization Practices (ACIP) Evidence to Recommendations (EtR) framework, a health care provider knowledge and attitudes survey was conducted during September 28-October 10, 2022, by the Healthcare and Public Perceptions of Immunizations Survey Collaborative before the October 2022 ACIP meeting. Among 751 provider respondents, two thirds agreed or strongly agreed with the policy option under consideration to expand the recommendations for the new 20-valent pneumococcal conjugate vaccine (PCV20) to adults who had only received the previously recommended 13-valent pneumococcal conjugate vaccine (PCV13). Gaps in providers' knowledge and perceived challenges to implementing recommendations were identified and were included in ACIP's EtR framework discussions in late October 2022 when ACIP updated the recommendations for PCV20 use in adults. Currently, use of PCV20 is recommended for certain adults who have previously received PCV13, in addition to those who have never received a pneumococcal conjugate vaccine. The survey findings indicate a need to increase provider awareness and implementation of pneumococcal vaccination recommendations and to provide tools to assist with patient-specific vaccination guidance. Resources available to address the challenges to implementing pneumococcal vaccination recommendations include the PneumoRecs VaxAdvisor mobile app and other CDC-developed tools, including summary documents and overviews of vaccination schedules and CDC's strategic framework to increase confidence in vaccines and reduce vaccine-preventable diseases, Vaccinate with Confidence.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Andrew M. Parker reports grants outside the submitted work from the National Institutes of Health, National Science Foundation, Department of Homeland Security, and Agency for Healthcare Research and Quality. Aaron M. Scherer reports grant funding from the National Institutes of Health. No other potential conflicts of interest were disclosed.
Figures
References
-
- Kobayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among U.S. adults and proposed policy options. Presented at the Advisory Committee on Immunization Practices meeting, Atlanta, GA; October 20, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-2...
-
- Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:109–17. 10.15585/mmwr.mm7104a1 - DOI - PMC - PubMed
-
- Tomczyk S, Bennett NM, Stoecker C, et al.; CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822–5. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

