Oxetanes in Drug Discovery Campaigns
- PMID: 37676858
- PMCID: PMC10544023
- DOI: 10.1021/acs.jmedchem.3c01101
Oxetanes in Drug Discovery Campaigns
Abstract
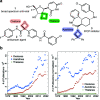
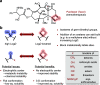
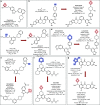
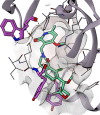
The oxetane ring is an emergent, underexplored motif in drug discovery that shows attractive properties such as low molecular weight, high polarity, and marked three-dimensionality. Oxetanes have garnered further interest as isosteres of carbonyl groups and as molecular tools to fine-tune physicochemical properties of drug compounds such as pKa, LogD, aqueous solubility, and metabolic clearance. This perspective highlights recent applications of oxetane motifs in drug discovery campaigns (2017-2022), with emphasis on the effect of the oxetane on medicinally relevant properties and on the building blocks used to incorporate the oxetane ring. Based on this analysis, we provide an overview of the potential benefits of appending an oxetane to a drug compound, as well as potential pitfalls, challenges, and future directions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Li J.; Eastgate M. D. Current Complexity: A Tool for Assessing the Complexity of Organic Molecules. Org. Biomol. Chem. 2015, 13, 7164–7176. 10.1039/C5OB00709G. - DOI - PubMed
- Caille S.; Cui S.; Faul M. M.; Mennen S. M.; Tedrow J. S.; Walker S. D. Molecular Complexity as a Driver for Chemical Process Innovation in the Pharmaceutical Industry. J. Org. Chem. 2019, 84, 4583–4603. 10.1021/acs.joc.9b00735. - DOI - PubMed
- Ivanenkov Y. A.; Zagribelnyy B. A.; Aladinskiy V. A. Are We Opening the Door to a New Era of Medicinal Chemistry or Being Collapsed to a Chemical Singularity?. J. Med. Chem. 2019, 62, 10026–10043. 10.1021/acs.jmedchem.9b00004. - DOI - PubMed
-
- Hann M. M.; Leach A. R.; Harper G. Molecular Complexity and Its Impact on the Probability of Finding Leads for Drug Discovery. J. Chem. Inf. Comput. Sci. 2001, 41, 856–864. 10.1021/ci000403i. - DOI - PubMed
- Clemons P. A.; Bodycombe N. E.; Carrinski H. A.; Wilson J. A.; Shamji A. F.; Wagner B. K.; Koehler A. N.; Schreiber S. L. Small Molecules of Different Origins Have Distinct Distributions of Structural Complexity That Correlate with Protein-Binding Profiles. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 18787–18792. 10.1073/pnas.1012741107. - DOI - PMC - PubMed
- Lovering F. Escape from Flatland 2: Complexity and Promiscuity. Medchemcomm 2013, 4, 515–519. 10.1039/c2md20347b. - DOI
-
- González-Medina M.; Prieto-Martínez F. D.; Naveja J. J.; Méndez-Lucio O.; El-Elimat T.; Pearce C. J.; Oberlies N. H.; Figueroa M.; Medina-Franco J. L. Chemoinformatic Expedition of the Chemical Space of Fungal Products. Future Med. Chem. 2016, 8, 1399–1412. 10.4155/fmc-2016-0079. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information

