Synthesis of copaiba (Copaifera officinalis) oil nanoemulsion and the potential against Zika virus: An in vitro study
- PMID: 37676868
- PMCID: PMC10484457
- DOI: 10.1371/journal.pone.0283817
Synthesis of copaiba (Copaifera officinalis) oil nanoemulsion and the potential against Zika virus: An in vitro study
Abstract
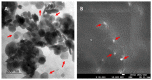
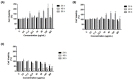
Zika virus (ZIKV) has spread all over the world since its major outbreak in 2015. This infection has been recognized as a major global health issue due to the neurological complications related to ZIKV infection, such as Guillain-Barré Syndrome and Zika virus Congenital Syndrome. Currently, there are no vaccines or specific treatments for ZIKV infection, which makes the development of specific therapies for its treatment very important. Several studies have been developed to analyze the potential of compounds against ZIKV, with the aim of finding new promising treatments. Herein, we evaluate the ability of a copaiba (Copaifera officinalis) oil nanoemulsion (CNE) to inhibit ZIKV. First, the highest non-cytotoxic concentration of 180 μg/mL was chosen since this concentration maintains 80% cell viability up to 96h after treatment with CNE in VERO cells resulted from MTT assay. The intracellular uptake assay was performed, and confirmed the internalization of the nanoemulsion in cells at all times analyzed. VERO cells were infected with ZIKV and simultaneously treated with CNE and the nanoformulation without oil (ENE) at the highest non-toxic concentration. The results evaluated by plaque assay revealed a viral inhibition of 80% for CNE and 70% for ENE. A dose-dependence assay revealed that the CNE treatment demonstrated a dose-dependent response in the viral RNA levels, whereas all ENE tested concentrations exhibited a similar degree of reduction. Taken together, our results suggest CNE as a promising nano-sized platform to be further studied for antiviral treatments.
Copyright: © 2023 Carvalho et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Resveratrol affects Zika virus replication in vitro.Sci Rep. 2019 Oct 4;9(1):14336. doi: 10.1038/s41598-019-50674-3. Sci Rep. 2019. PMID: 31586088 Free PMC article.
-
Exploring the antiviral potential of justicidin B and four glycosylated lignans from Phyllanthus brasiliensis against Zika virus: A promising pharmacological approach.Phytomedicine. 2024 Jan;123:155197. doi: 10.1016/j.phymed.2023.155197. Epub 2023 Nov 7. Phytomedicine. 2024. PMID: 37952409
-
Identification of Inhibitors of ZIKV Replication.Viruses. 2020 Sep 18;12(9):1041. doi: 10.3390/v12091041. Viruses. 2020. PMID: 32961956 Free PMC article.
-
Favipiravir and Ribavirin Inhibit Replication of Asian and African Strains of Zika Virus in Different Cell Models.Viruses. 2018 Feb 9;10(2):72. doi: 10.3390/v10020072. Viruses. 2018. PMID: 29425176 Free PMC article.
-
NS2B-NS3 protease inhibitors as promising compounds in the development of antivirals against Zika virus: A systematic review.J Med Virol. 2022 Feb;94(2):442-453. doi: 10.1002/jmv.27386. Epub 2021 Oct 20. J Med Virol. 2022. PMID: 34636434
Cited by
-
Synthesis and Physicochemical Stability of a Copaiba Balsam Oil (Copaifera sp.) Nanoemulsion and Prospecting of Toxicological Effects on the Nematode Caenorhabditis elegans.ACS Omega. 2024 Sep 6;9(37):39100-39118. doi: 10.1021/acsomega.4c05930. eCollection 2024 Sep 17. ACS Omega. 2024. PMID: 39310144 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

