Trichostatin A for Efficient CRISPR-Cas9 Gene Editing of Human Pluripotent Stem Cells
- PMID: 37676985
- PMCID: PMC10611976
- DOI: 10.1089/crispr.2023.0033
Trichostatin A for Efficient CRISPR-Cas9 Gene Editing of Human Pluripotent Stem Cells
Abstract
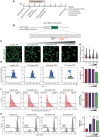
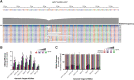
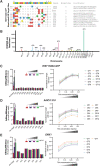
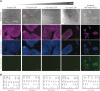
Genome-edited human-induced pluripotent stem cells (iPSCs) have broad applications in disease modeling, drug discovery, and regenerative medicine. Despite the development of clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 system, the gene editing process is inefficient and can take several weeks to months to generate edited iPSC clones. We developed a strategy to improve the efficiency of the iPSC gene editing process via application of a small-molecule, trichostatin A (TSA), a Class I and II histone deacetylase inhibitor. We observed that TSA decreased global chromatin condensation and further resulted in increased gene-editing efficiency of iPSCs by twofold to fourfold while concurrently ensuring no increased off-target effects. The edited iPSCs could be clonally expanded while maintaining genomic integrity and pluripotency. The rapid generation of therapeutically relevant gene-edited iPSCs could be enabled by these findings.
Conflict of interest statement
K.M. and K.S. have filed a patent application on this work. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Determining the Pathogenicity of a Genomic Variant of Uncertain Significance Using CRISPR/Cas9 and Human-Induced Pluripotent Stem Cells.Circulation. 2018 Dec 4;138(23):2666-2681. doi: 10.1161/CIRCULATIONAHA.117.032273. Circulation. 2018. PMID: 29914921 Free PMC article.
-
A simple, quick, and efficient CRISPR/Cas9 genome editing method for human induced pluripotent stem cells.Acta Pharmacol Sin. 2020 Nov;41(11):1427-1432. doi: 10.1038/s41401-020-0452-0. Epub 2020 Jun 18. Acta Pharmacol Sin. 2020. PMID: 32555510 Free PMC article.
-
CRISPR/Cas9 Genome Editing: A Promising Tool for Therapeutic Applications of Induced Pluripotent Stem Cells.Curr Stem Cell Res Ther. 2018;13(4):243-251. doi: 10.2174/1574888X13666180214124800. Curr Stem Cell Res Ther. 2018. PMID: 29446747 Review.
-
Gene Editing in Human Induced Pluripotent Stem Cells Using Doxycycline-Inducible CRISPR-Cas9 System.Methods Mol Biol. 2022;2454:755-773. doi: 10.1007/7651_2021_348. Methods Mol Biol. 2022. PMID: 33830454 Free PMC article.
-
Induced Pluripotent Stem Cells in the Era of Precise Genome Editing.Curr Stem Cell Res Ther. 2024;19(3):307-315. doi: 10.2174/1574888X18666230307115326. Curr Stem Cell Res Ther. 2024. PMID: 36880183 Review.
Cited by
-
Current Strategies for Increasing Knock-In Efficiency in CRISPR/Cas9-Based Approaches.Int J Mol Sci. 2024 Feb 20;25(5):2456. doi: 10.3390/ijms25052456. Int J Mol Sci. 2024. PMID: 38473704 Free PMC article. Review.
-
Molecular mechanisms and functions of protein acetylation in sepsis and sepsis-associated organ dysfunction.Cell Mol Biol Lett. 2025 Jul 26;30(1):91. doi: 10.1186/s11658-025-00773-z. Cell Mol Biol Lett. 2025. PMID: 40713493 Free PMC article. Review.
-
Comparative epigenetics of domestic animals: focusing on DNA accessibility and its impact on gene regulation and traits.J Vet Sci. 2025 Jan;26(1):e9. doi: 10.4142/jvs.24259. J Vet Sci. 2025. PMID: 39901471 Free PMC article. Review.
-
Controlling CRISPR-Cas9 genome editing in human cells using a molecular glue degrader.Mol Ther Nucleic Acids. 2025 Jul 21;36(3):102640. doi: 10.1016/j.omtn.2025.102640. eCollection 2025 Sep 9. Mol Ther Nucleic Acids. 2025. PMID: 40799508 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

