Efficacy and Safety of the Melanocortin Pan-Agonist PL9643 in a Phase 2 Study of Patients with Dry Eye Disease
- PMID: 37677000
- PMCID: PMC10654643
- DOI: 10.1089/jop.2023.0056
Efficacy and Safety of the Melanocortin Pan-Agonist PL9643 in a Phase 2 Study of Patients with Dry Eye Disease
Abstract
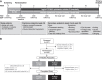
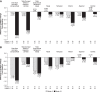
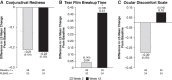
Purpose: The melanocortin receptor pan-agonist PL9643, a potential therapy for ocular diseases, was investigated in a phase 2, 12-week study in patients with dry eye disease (DED). Methods: This was a placebo-controlled study evaluating efficacy and safety of thrice-daily PL9643. Placebo (vehicle) was similar to tears. Primary endpoints were intra-patient changes in inferior corneal fluorescein staining and ocular discomfort after 12 weeks. Secondary endpoints were changes in additional DED signs or symptoms. Multiple secondary endpoints were not adjusted for multiplicity. Patients with moderate or severe DED were analyzed in addition to the overall intent-to-treat (ITT) population. Results: In the ITT population (n = 160) the PL9643 group did not demonstrate significant treatment difference versus placebo at week 12/day 85 for the primary endpoints (P > 0.05). In patients with moderate or severe DED (n = 53), PL9643 treatment demonstrated either nominally significant (P < 0.05) or trending (P < 0.1) improvement over placebo in mean change from baseline at week 12/day 85 in several sign endpoints, including fluorescein staining in inferior, superior, corneal sum, and total sum regions; Lissamine Green staining in temporal, nasal, conjunctival sum, and total sum regions; and tear film breakup time. Conjunctival redness also showed (nonsignificant) improvement at week 12/day 85. There were no drug-related adverse events (AEs) and no drug-related discontinuations. Conclusions: PL9643 showed no significant efficacy for the ITT population; however, efficacy results across several signs and symptoms in the subpopulation of moderate to severe DED patients, the low number of ocular AEs, and no tolerability issues suggest that PL9643 shows promise as a therapeutic for DED. Clinical Trial Registration number: NCT04268069.
Keywords: PL9643; conjunctival staining; dry eye disease; efficacy; melanocortin receptor agonist; tear film breakup time.
Conflict of interest statement
D.E. received financial support from Alcon, Allergan, AxeroVision, Bausch + Lomb, Hovione, Kala, Novaliq, Novartis, Ocular Therapeutix, and Vistakon. K.K. was an independent contractor at Ora, Inc., throughout the duration of the study. G.O. and M.W. were employees of Ora, Inc., throughout the duration of the study. G.T. received financial support from Mitotech, Kowa, Aldeyra, Topivert, Brim, Palatin, Oyster Point, Allergan, Aerie, Aurinia, ReGenTree, Novaliq, HanAll, and Ora, Inc. P.V. has nothing to disclose. E.B.M. received financial support from Allergan, Aldeyra, Aurinia, HanAll, Mallinckrodt, Mitotech, Nicox, Novaliq, Orasis, Ocular Therapeutix, Palatin, ReGenTree, Santen, and Topivert. J.W., J.D., R.J., S.T.W., and C.S. are employees of Palatin Technologies, Inc.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
