Atrial fibrillation-associated electrical remodelling in human induced pluripotent stem cell-derived atrial cardiomyocytes: a novel pathway for antiarrhythmic therapy development
- PMID: 37677054
- PMCID: PMC10730244
- DOI: 10.1093/cvr/cvad143
Atrial fibrillation-associated electrical remodelling in human induced pluripotent stem cell-derived atrial cardiomyocytes: a novel pathway for antiarrhythmic therapy development
Abstract
Aims: Atrial fibrillation (AF) is associated with tachycardia-induced cellular electrophysiology alterations which promote AF chronification and treatment resistance. Development of novel antiarrhythmic therapies is hampered by the absence of scalable experimental human models that reflect AF-associated electrical remodelling. Therefore, we aimed to assess if AF-associated remodelling of cellular electrophysiology can be simulated in human atrial-like cardiomyocytes derived from induced pluripotent stem cells in the presence of retinoic acid (iPSC-aCM), and atrial-engineered human myocardium (aEHM) under short term (24 h) and chronic (7 days) tachypacing (TP).
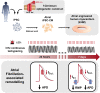
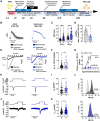
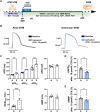
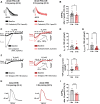
Methods and results: First, 24-h electrical pacing at 3 Hz was used to investigate whether AF-associated remodelling in iPSC-aCM and aEHM would ensue. Compared to controls (24 h, 1 Hz pacing) TP-stimulated iPSC-aCM presented classical hallmarks of AF-associated remodelling: (i) decreased L-type Ca2+ current (ICa,L) and (ii) impaired activation of acetylcholine-activated inward-rectifier K+ current (IK,ACh). This resulted in action potential shortening and an absent response to the M-receptor agonist carbachol in both iPSC-aCM and aEHM subjected to TP. Accordingly, mRNA expression of the channel-subunit Kir3.4 was reduced. Selective IK,ACh blockade with tertiapin reduced basal inward-rectifier K+ current only in iPSC-aCM subjected to TP, thereby unmasking an agonist-independent constitutively active IK,ACh. To allow for long-term TP, we developed iPSC-aCM and aEHM expressing the light-gated ion-channel f-Chrimson. The same hallmarks of AF-associated remodelling were observed after optical-TP. In addition, continuous TP (7 days) led to (i) increased amplitude of inward-rectifier K+ current (IK1), (ii) hyperpolarization of the resting membrane potential, (iii) increased action potential-amplitude and upstroke velocity as well as (iv) reversibly impaired contractile function in aEHM.
Conclusions: Classical hallmarks of AF-associated remodelling were mimicked through TP of iPSC-aCM and aEHM. The use of the ultrafast f-Chrimson depolarizing ion channel allowed us to model the time-dependence of AF-associated remodelling in vitro for the first time. The observation of electrical remodelling with associated reversible contractile dysfunction offers a novel platform for human-centric discovery of antiarrhythmic therapies.
Keywords: Action potential; Atrial fibrillation; Ion channel; Optogenetics; Stem cells.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: W.H.Z. is founder and advisor of Repairon GmbH and myriamed GmbH. M.T. is advisor of Repairon GmbH and myriamed GmbH. myriamed GmbH commercializes iPSC-based cell and tissue models for drug discovery. M.Rap. is an employee of Nanion Technologies GmbH. This manuscript was handled by Consulting Editor David Eisner.
Figures




References
-
- Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. - PubMed
-
- Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XHT, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. - PMC - PubMed
-
- Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 1999;85:428–436. - PubMed
-
- Christ T, Boknik P, Wöhrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation 2004;110:2651–2657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

