Hyperoxia Increases Kidney Injury During Renal Ischemia and Reperfusion in Mice
- PMID: 37678264
- PMCID: PMC10592523
- DOI: 10.1213/ANE.0000000000006600
Hyperoxia Increases Kidney Injury During Renal Ischemia and Reperfusion in Mice
Abstract
Background: Renal ischemia and reperfusion (IR) contribute to perioperative acute kidney injury, and oxygen is a key regulator of this process. We hypothesized that oxygen administration during surgery and renal IR would impact postoperative kidney function and injury in mice.
Methods: Mice were anesthetized, intubated, and mechanically ventilated with a fraction of inspired oxygen (F io2 ) 0.10 (hypoxia), 0.21 (normoxia), 0.60 (moderate hyperoxia), or 1.00 (severe hyperoxia) during 67 minutes of renal IR or sham IR surgery. Additional mice were treated before IR or sham IR surgery with 50 mg/kg tempol, a superoxide scavenger. At 24 hours, mice were sacrificed, and blood and kidney collected. We assessed and compared kidney function and injury across groups by measuring blood urea nitrogen (BUN, primary end point), renal histological injury, renal expression of neutrophil gelatinase-associated lipocalin (NGAL), and renal heme oxygenase 1 ( Ho-1 ), peroxisome proliferator-activated receptor gamma coactivator 1-α ( Pgc1-α ), and glutathione peroxidase 4 ( Gpx-4 ) transcripts, to explore potential mechanisms of any effect of oxygen.
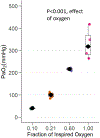
Results: Hyperoxia and hypoxia during renal IR surgery decreased renal function and increased kidney injury compared to normoxia. Baseline median (interquartile range) BUN was 22.2 mg/dL (18.4-26.0), and 24 hours after IR surgery, BUN was 17.5 mg/dL (95% confidence interval [CI], 1.3-38.4; P = .034) higher in moderate hyperoxia-treated animals, 51.8 mg/dL (95% CI, 24.9-74.8; P < .001) higher in severe hyperoxia-treated animals, and 64.9 mg/dL (95% CI, 41.2-80.3; P < .001) higher in hypoxia-treated animals compared to animals treated with normoxia ( P < .001, overall effect of hyperoxia). Hyperoxia-induced injury, but not hypoxia-induced injury, was attenuated by pretreatment with tempol. Histological injury scores, renal NGAL staining, and renal transcription of Ho-1 and suppression of Pgc1- α followed the same pattern as BUN, in relation to the effects of oxygen treatment.
Conclusions: In this controlled preclinical study of oxygen treatment during renal IR surgery, hyperoxia and hypoxia impaired renal function, increased renal injury, and impacted expression of genes that affect mitochondrial biogenesis and antioxidant response. These results might have implications for patients during surgery when high concentrations of oxygen are frequently administered, especially in cases involving renal IR.
Copyright © 2023 International Anesthesia Research Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Khan S, Cleveland R, Koch C, Schelling J. Hypoxia induces renal tubular epithelial cell apoptosis in chronic renal disease. Laboratory investigation; a journal of technical methods and pathology. 1999 Sep 1999;79(9) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL164909/HL/NHLBI NIH HHS/United States
- R35 GM145375/GM/NIGMS NIH HHS/United States
- K23 GM129662/GM/NIGMS NIH HHS/United States
- UC2 DK126122/DK/NIDDK NIH HHS/United States
- R13 DK132844/DK/NIDDK NIH HHS/United States
- R35GM145375/NH/NIH HHS/United States
- R01DK132844/NH/NIH HHS/United States
- R01 HL144943/HL/NHLBI NIH HHS/United States
- T32GM108554/NH/NIH HHS/United States
- UC2DK126122/NH/NIH HHS/United States
- K23GM129662/NH/NIH HHS/United States
- R01 GM112871/GM/NIGMS NIH HHS/United States
- T32 GM108554/GM/NIGMS NIH HHS/United States
- R01 HL157583/HL/NHLBI NIH HHS/United States
- R01GM112871/NH/NIH HHS/United States
- R01HL157583/NH/NIH HHS/United States
- R01HL164909/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

