Uterine Fluid Extracellular Vesicles Proteome Is Altered During the Estrous Cycle
- PMID: 37678639
- PMCID: PMC10641272
- DOI: 10.1016/j.mcpro.2023.100642
Uterine Fluid Extracellular Vesicles Proteome Is Altered During the Estrous Cycle
Abstract
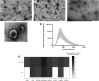
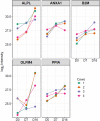
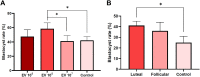
Uterine environment is tightly and finely regulated via various signaling pathways mediated through endocrine, exocrine, autocrine, juxtacrine, and paracrine mechanisms. In utero signaling processes are paramount for normal and abnormal physiology which involves cell to cell, cells to gametes, cells to embryo, and even interkingdom communications due to presence of uterine microbiota. Extracellular vesicles (EVs) in the uterine fluid (UF) and their cargo components are known to be mediators of in utero signaling and communications. Interestingly, the changes in UF-EV proteome during the bovine estrous cycle and the effects of these differentially enriched proteins on embryo development are yet to be fully discovered. In this study, shotgun quantitative proteomics-based mass spectrometry was employed to compare UF-EV proteomes at day 0, 7, and 16 of the estrous cycle to understand the estrous cycle-dependent dynamics. Furthermore, different phase UF-EVs were supplemented in embryo cultures to evaluate their impact on embryo development. One hundred fifty-nine UF-EV proteins were differentially enriched at different time points indicating the UF-EV proteome is cycle-dependent. Overall, many identified pathways are important for normal uterine functions, early embryo development, and its nutritional needs, such as antioxidant activity, cell morphology and cycle, cellular homeostasis, cell adhesion, and carbohydrate metabolic process. Furthermore, the luteal phase UF-EVs supplementation increased in vitro blastocyst rates from 25.0 ± 5.9% to 41.0 ± 4.0% (p ≤ 0.05). Our findings highlight the importance of bovine UF-EV in uterine communications throughout the estrous cycle. Interestingly, comparison of hormone-synchronized EV proteomes to natural cycle UF-EVs indicated shift of signaling. Finally, UF-EVs can be used to improve embryo production in vitro.
Keywords: bovine uterine fluid; embryo culture; extracellular vesicles; proteome.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures




References
-
- Forde N., Beltman M.E., Lonergan P., Diskin M., Roche J.F., Crowe M.A. Oestrous cycles in Bos taurus cattle. Anim. Reprod. Sci. 2011;124:163–169. - PubMed
-
- Faulkner S., Elia G., O’Boyle P., Dunn M., Morris D. Composition of the bovine uterine proteome is associated with stage of cycle and concentration of systemic progesterone. Proteomics. 2013;13:3333–3353. - PubMed
-
- Moran E.T. Nutrition of the developing embryo and hatchling. Poult. Sci. 2007;86:1043–1049. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

