Protocol for the YORKSURe prospective multistage study testing the feasibility for early detection of bladder cancer in populations with high disease-specific mortality risk
- PMID: 37678944
- PMCID: PMC10496676
- DOI: 10.1136/bmjopen-2023-076612
Protocol for the YORKSURe prospective multistage study testing the feasibility for early detection of bladder cancer in populations with high disease-specific mortality risk
Abstract
Introduction: Around 25% of patients with bladder cancer (BCa) present with invasive disease. Non-randomised studies of population-based screening have suggested reductions in BCa-specific mortality are possible through earlier detection. The low prevalence of lethal disease in the general population means screening is not cost-effective and there is no consensus on the best strategy. Yorkshire has some of the highest mortality rates from BCa in England. We aim to test whether population screening in a region of high mortality risk will lead to a downward stage-migration of aggressive BCa, improved survival and is cost-effective.
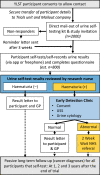
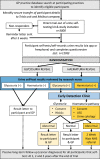
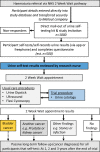
Methods and analysis: YORKSURe is a tiered, randomised, multicohort study to test the feasibility of a large BCa screening randomised controlled trial. In three parallel cohorts, participants will self-test urine (at home) up to six times. Results are submitted via a mobile app or freephone. Those with a positive result will be invited for further investigation at community-based early detection clinics or within usual National Health Service (NHS) pathways. In Cohort 1, we will post self-testing kits to research engaged participants (n=2000) embedded within the Yorkshire Lung Screening Trial. In Cohort 2, we will post self-testing kits to 3000 invitees. Cohort 2 participants will be randomised between haematuria and glycosuria testing using a reveal/conceal design. In Cohort 3, we will post self-testing kits to 500 patients within the NHS pathway for investigation of haematuria. Our primary outcomes are rates of recruitment and randomisation, rates of positive test and acceptability of the design. The study is currently recruiting and scheduled to finish in June 2023.
Ethics and dissemination: The study has received the following approvals: London Riverside Research Ethics Committee (22/LO/0018) and Health Research Authority Confidentiality Advisory Group (20/CAG/0009). Results will be made available to providers and researchers via publicly accessible scientific journals.
Trial registration number: ISRCTN34273159.
Keywords: bladder disorders; mass screening; urological tumours.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: JC has received reimbursement for consultancy from Astra Zeneca, BMS, Ipsen, Janssen and Roche, speaker fees from BMS, Ipsen, MSD, Nucleix and Roche, honoraria for membership of advisory boards from Astra Zeneca, Ferring, Roche and Janssen and research funding from Roche. PS is a paid member of the Scientific Advisory Board of GRAIL and the medical advisory board of NSV. The remaining authors declare no potential conflicts of interest.
Figures
References
-
- Catto JWF, Khetrapal P, Ricciardi F, et al. Effect of robot-assisted radical cystectomy with Intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. JAMA 2022;327:2092–103. 10.1001/jama.2022.7393 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
