MRI pulmonary artery flow detects lung vascular pathology in preterms with lung disease
- PMID: 37678954
- PMCID: PMC10749508
- DOI: 10.1183/13993003.02445-2022
MRI pulmonary artery flow detects lung vascular pathology in preterms with lung disease
Abstract
Background: Pulmonary vascular disease (PVD) affects the majority of preterm neonates with bronchopulmonary dysplasia (BPD) and significantly determines long-term mortality through undetected progression into pulmonary hypertension. Our objectives were to associate characteristics of pulmonary artery (PA) flow and cardiac function with BPD-associated PVD near term using advanced magnetic resonance imaging (MRI) for improved risk stratification.
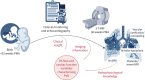
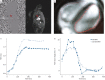
Methods: Preterms <32 weeks postmenstrual age (PMA) with/without BPD were clinically monitored including standard echocardiography and prospectively enrolled for 3 T MRI in spontaneous sleep near term (AIRR (Attention to Infants at Respiratory Risks) study). Semi-manual PA flow quantification (phase-contrast MRI; no BPD n=28, mild BPD n=35 and moderate/severe BPD n=25) was complemented by cardiac function assessment (cine MRI).
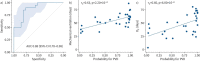
Results: We identified abnormalities in PA flow and cardiac function, i.e. increased net forward volume right/left ratio, decreased mean relative area change and pathological right end-diastolic volume, to sensitively detect BPD-associated PVD while correcting for PMA (leave-one-out area under the curve 0.88, sensitivity 0.80 and specificity 0.81). We linked these changes to increased right ventricular (RV) afterload (RV-arterial coupling (p=0.02), PA mid-systolic notching (t2; p=0.015) and cardiac index (p=1.67×10-8)) and correlated echocardiographic findings. Identified in moderate/severe BPD, we successfully applied the PA flow model in heterogeneous mild BPD cases, demonstrating strong correlation of PVD probability with indicators of BPD severity, i.e. duration of mechanical ventilation (rs=0.63, p=2.20×10-4) and oxygen supplementation (rs=0.60, p=6.00×10-4).
Conclusions: Abnormalities in MRI PA flow and cardiac function exhibit significant, synergistic potential to detect BPD-associated PVD, advancing the possibilities of risk-adapted monitoring.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: No authors have any potential conflicts of interest to disclose.
Figures


Comment in
-
Having new eyes: MRI for visualisation of pulmonary vascular disease and prediction of bronchopulmonary dysplasia severity.Eur Respir J. 2023 Dec 21;62(6):2302041. doi: 10.1183/13993003.02041-2023. Print 2023 Dec. Eur Respir J. 2023. PMID: 38128954 No abstract available.
References
-
- Hilgendorff A, Apitz C, Bonnet D, et al. . Pulmonary hypertension associated with acute or chronic lung diseases in the preterm and term neonate and infant. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102: Suppl. 2, ii49–ii56. doi:10.1136/heartjnl-2015-308591 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
