A meta-analysis of epitopes in prostate-specific antigens identifies opportunities and knowledge gaps
- PMID: 37679223
- PMCID: PMC11017785
- DOI: 10.1016/j.humimm.2023.08.145
A meta-analysis of epitopes in prostate-specific antigens identifies opportunities and knowledge gaps
Abstract
Background: The Cancer Epitope Database and Analysis Resource (CEDAR) is a newly developed repository of cancer epitope data from peer-reviewed publications, which includes epitope-specific T cell, antibody, and MHC ligand assays. Here we focus on prostate cancer as our first cancer category to demonstrate the capabilities of CEDAR, and to shed light on the advances of epitope-related prostate cancer research.
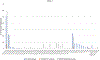
Results: The meta-analysis focused on a subset of data describing epitopes from 8 prostate-specific (PS) antigens. A total of 460 epitopes were associated with these proteins, 187 T cell, 109B cell, and 271 MHC ligand epitopes. The number of epitopes was not correlated with the length of the protein; however, we found a significant positive correlation between the number of references per specific PS antigen and the number of reported epitopes. Forty-four different class I and 27 class II restrictions were found, with the most epitopes described for HLA-A*02:01 and HLA-DRB1*01:01. Cytokine assays were mostly limited to IFNg assays and a very limited number of tetramer assays were performed. Monoclonal and polyclonal B cell responses were balanced, with the highest number of epitopes studied in ELISA/Western blot assays. Additionally, epitopes were generically described as associated with prostate cancer, with little granularity specifying diseases state. We found that in vivo and tumor recognition assays were sparse, and the number of epitopes with annotated B/T cell receptor information were limited. Potential immunodominant regions were identified by the use of the ImmunomeBrowser tool.
Conclusion: CEDAR provides a comprehensive repository of epitopes related to prostate-specific antigens. This inventory of epitope data with its wealth of searchable T cell, B cell and MHC ligand information provides a useful tool for the scientific community. At the same time, we identify significant knowledge gaps that could be addressed by experimental analysis.
Keywords: Antibodies; CEDAR; Cancer; Database; Epitope; MHC ligand; Prostate; T cell.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

