Asarinin attenuates bleomycin-induced pulmonary fibrosis by activating PPARγ
- PMID: 37679587
- PMCID: PMC10485066
- DOI: 10.1038/s41598-023-41933-5
Asarinin attenuates bleomycin-induced pulmonary fibrosis by activating PPARγ
Abstract
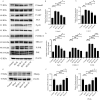
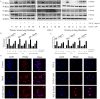
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive interstitial lung disease that lacks effective treatment modalities. Once patients are diagnosed with IPF, their median survival is approximately 3-5 years. PPARγ is an important target for the prevention and treatment of pulmonary fibrosis. Asarinin is a lignan compound that can be extracted from food plant Asarum heterotropoides. In this study, we investigated the therapeutic effects of asarinin in a pulmonary fibrosis model constructed using bleomycin in mice and explored the underlying mechanisms. Intraperitoneal administration of asarinin to mice with pulmonary fibrosis showed that asarinin effectively attenuated pulmonary fibrosis, and this effect was significantly inhibited by the PPARγ inhibitor GW9662. Asarinin inhibited TGF-β1-induced fibroblast-to-myofibroblast transition in vitro, while GW9662 and PPARγ gene silencing significantly inhibited this effect. In addition, asarinin inhibited not only the canonical Smad pathway of TGF-β but also the non-canonical AKT and MAPK pathways by activating PPARγ. Our study demonstrates that asarinin can be used as a therapeutic agent for pulmonary fibrosis, and that PPARγ is its key target.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

