Supraphysiologic doses of 17β-estradiol aggravate depression-like behaviors in ovariectomized mice possibly via regulating microglial responses and brain glycerophospholipid metabolism
- PMID: 37679787
- PMCID: PMC10485970
- DOI: 10.1186/s12974-023-02889-5
Supraphysiologic doses of 17β-estradiol aggravate depression-like behaviors in ovariectomized mice possibly via regulating microglial responses and brain glycerophospholipid metabolism
Abstract
Background: 17β-Estradiol (E2) is generally considered neuroprotective in humans. However, the current clinical use of estrogen replacement therapy (ERT) is based on the physiological dose of E2 to treat menopausal syndrome and has limited therapeutic efficacy. The efficacy and potential toxicity of superphysiological doses of ERT for menopausal neurodegeneration are unknown.
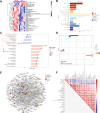
Methods: In this study, we investigated the effect of E2 with a supraphysiologic dose (0.5 mg/kg, sE2) on the treatment of menopausal mouse models established by ovariectomy. We performed the open field, Y-maze spontaneous alternation, forced swim tests, and sucrose preference test to investigate behavioral alterations. Subsequently, the status of microglia and neurons was detected by immunohistochemistry, HE staining, and Nissl staining, respectively. Real-time PCR was used to detect neuroinflammatory cytokines in the hippocampus and cerebral cortex. Using mass spectrometry proteomics platform and LC-MS/ MS-based metabolomics platform, proteins and metabolites in brain tissues were extracted and analyzed. BV2 and HT22 cell lines and primary neurons and microglia were used to explore the underlying molecular mechanisms in vitro.
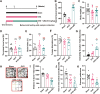
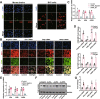
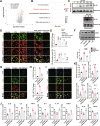
Results: sE2 aggravated depression-like behavior in ovariectomized mice, caused microglia response, and increased proinflammatory cytokines in the cerebral cortex and hippocampus, as well as neuronal damage and glycerophospholipid metabolism imbalance. Subsequently, we demonstrated that sE2 induced the pro-inflammatory phenotype of microglia through ERα/NF-κB signaling pathway and downregulated the expression of cannabinoid receptor 1 in neuronal cells, which were important in the pathogenesis of depression.
Conclusion: These data suggest that sE2 may be nonhelpful or even detrimental to menopause-related depression, at least partly, by regulating microglial responses and glycerophospholipid metabolism.
Keywords: 17β-Estradiol; Depression; Estrogen replacement therapy; Glycerophospholipid metabolism; Menopausal syndrome; Microglial.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Neuroprotective effects of vitamin D and 17ß-estradiol against ovariectomy-induced neuroinflammation and depressive-like state: Role of the AMPK/NF-κB pathway.Int Immunopharmacol. 2020 Sep;86:106734. doi: 10.1016/j.intimp.2020.106734. Epub 2020 Jun 27. Int Immunopharmacol. 2020. PMID: 32604067
-
Gastrodin programs an Arg-1+ microglial phenotype in hippocampus to ameliorate depression- and anxiety-like behaviors via the Nrf2 pathway in mice.Phytomedicine. 2023 May;113:154725. doi: 10.1016/j.phymed.2023.154725. Epub 2023 Feb 26. Phytomedicine. 2023. PMID: 36867963
-
Lipopolysaccharide-induced depression is associated with estrogen receptor-α/SIRT1/NF-κB signaling pathway in old female mice.Neurochem Int. 2021 Sep;148:105097. doi: 10.1016/j.neuint.2021.105097. Epub 2021 Jun 11. Neurochem Int. 2021. PMID: 34119591
-
Shaping Microglial Phenotypes Through Estrogen Receptors: Relevance to Sex-Specific Neuroinflammatory Responses to Brain Injury and Disease.J Pharmacol Exp Ther. 2020 Oct;375(1):223-236. doi: 10.1124/jpet.119.264598. Epub 2020 Jun 8. J Pharmacol Exp Ther. 2020. PMID: 32513838 Review.
-
Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease.Am J Med. 1997 Sep 22;103(3A):19S-25S. doi: 10.1016/s0002-9343(97)00260-x. Am J Med. 1997. PMID: 9344403 Review.
Cited by
-
Sini San ameliorates lipid metabolism in hyperprolactinemia rat with liver-depression.Curr Res Food Sci. 2024 Sep 14;9:100853. doi: 10.1016/j.crfs.2024.100853. eCollection 2024. Curr Res Food Sci. 2024. PMID: 39328388 Free PMC article.
-
Estrogen-immuno-neuromodulation disorders in menopausal depression.J Neuroinflammation. 2024 Jun 19;21(1):159. doi: 10.1186/s12974-024-03152-1. J Neuroinflammation. 2024. PMID: 38898454 Free PMC article. Review.
-
Rutin Attenuates Gentamycin-induced Hair Cell Injury in the Zebrafish Lateral Line via Suppressing STAT1.Mol Neurobiol. 2024 Nov;61(11):9548-9561. doi: 10.1007/s12035-024-04179-4. Epub 2024 Apr 24. Mol Neurobiol. 2024. PMID: 38653908
-
Santacruzamate A Alleviates Pain and Pain-Related Adverse Emotions through the Inhibition of Microglial Activation in the Anterior Cingulate Cortex.ACS Pharmacol Transl Sci. 2024 Feb 16;7(4):1002-1012. doi: 10.1021/acsptsci.3c00282. eCollection 2024 Apr 12. ACS Pharmacol Transl Sci. 2024. PMID: 38633586 Free PMC article.
-
Daphnetin Ameliorates Neuropathic Pain via Regulation of Microglial Responses and Glycerophospholipid Metabolism in the Spinal Cord.Pharmaceuticals (Basel). 2024 Jun 16;17(6):789. doi: 10.3390/ph17060789. Pharmaceuticals (Basel). 2024. PMID: 38931456 Free PMC article.
References
-
- Slopien R, Pluchino N, Warenik-Szymankiewicz A, Sajdak S, Luisi M, Drakopoulos P, Genazzani A. Correlation between allopregnanolone levels and depressive symptoms during late menopausal transition and early postmenopause. Gynecol Endocrinol. 2018;34(2):144–147. doi: 10.1080/09513590.2017.1371129. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

