Combining old and new concepts in targeting telomerase for cancer therapy: transient, immediate, complete and combinatory attack (TICCA)
- PMID: 37679807
- PMCID: PMC10483736
- DOI: 10.1186/s12935-023-03041-2
Combining old and new concepts in targeting telomerase for cancer therapy: transient, immediate, complete and combinatory attack (TICCA)
Abstract
Telomerase can overcome replicative senescence by elongation of telomeres but is also a specific element in most cancer cells. It is expressed more vastly than any other tumor marker. Telomerase as a tumor target inducing replicative immortality can be overcome by only one other mechanism: alternative lengthening of telomeres (ALT). This limits the probability to develop resistance to treatments. Moreover, telomerase inhibition offers some degree of specificity with a low risk of toxicity in normal cells. Nevertheless, only one telomerase antagonist reached late preclinical studies. The underlying causes, the pitfalls of telomerase-based therapies, and future chances based on recent technical advancements are summarized in this review. Based on new findings and approaches, we propose a concept how long-term survival in telomerase-based cancer therapies can be significantly improved: the TICCA (Transient Immediate Complete and Combinatory Attack) strategy.
Keywords: Cancer; Cancer therapy; TICCA; Telomerase; Telomere; Transient immediate complete and combinatory attack.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
There are no competing interests ber Haj Ali and Michal Walter wrote this review.
Figures
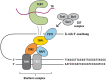
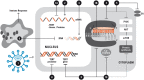
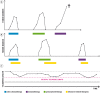
Similar articles
-
Therapeutic Targeting of Telomerase.Genes (Basel). 2016 Jul 21;7(7):39. doi: 10.3390/genes7070039. Genes (Basel). 2016. PMID: 27455328 Free PMC article. Review.
-
Telomere elongation via alternative lengthening of telomeres (ALT) and telomerase activation in primary metastatic medulloblastoma of childhood.J Neurooncol. 2019 May;142(3):435-444. doi: 10.1007/s11060-019-03127-w. Epub 2019 Mar 4. J Neurooncol. 2019. PMID: 30830680
-
Telomere maintenance mechanisms in cancer: telomerase, ALT or lack thereof.Curr Opin Genet Dev. 2020 Feb;60:1-8. doi: 10.1016/j.gde.2020.01.002. Epub 2020 Feb 27. Curr Opin Genet Dev. 2020. PMID: 32114293 Review.
-
Functional Loss of ATRX and TERC Activates Alternative Lengthening of Telomeres (ALT) in LAPC4 Prostate Cancer Cells.Mol Cancer Res. 2019 Dec;17(12):2480-2491. doi: 10.1158/1541-7786.MCR-19-0654. Epub 2019 Oct 14. Mol Cancer Res. 2019. PMID: 31611308 Free PMC article.
-
Overcoming the immortality of tumour cells by telomere and telomerase based cancer therapeutics--current status and future prospects.Eur J Cancer. 2005 May;41(7):971-9. doi: 10.1016/j.ejca.2004.11.024. Eur J Cancer. 2005. PMID: 15862745 Review.
Cited by
-
Promising new drugs and therapeutic approaches for treatment of ovarian cancer-targeting the hallmarks of cancer.BMC Med. 2025 Jan 6;23(1):10. doi: 10.1186/s12916-024-03826-w. BMC Med. 2025. PMID: 39762846 Free PMC article. Review.
-
Telomere Length and Telomerase Activity as Potential Biomarkers for Gastrointestinal Cancer.Cancers (Basel). 2024 Oct 1;16(19):3370. doi: 10.3390/cancers16193370. Cancers (Basel). 2024. PMID: 39409990 Free PMC article. Review.
-
Beginning at the ends: telomere and telomere-based cancer therapeutics.Mol Genet Genomics. 2024 Dec 6;300(1):1. doi: 10.1007/s00438-024-02206-6. Mol Genet Genomics. 2024. PMID: 39638969 Review.
-
Preparation and anticancer activity of telomerase inhibitor TAT-LPTS39 polypeptide.Transl Cancer Res. 2024 Sep 30;13(9):4625-4638. doi: 10.21037/tcr-24-792. Epub 2024 Sep 27. Transl Cancer Res. 2024. PMID: 39430831 Free PMC article.
-
From Crypts to Cancer: A Holistic Perspective on Colorectal Carcinogenesis and Therapeutic Strategies.Int J Mol Sci. 2024 Aug 30;25(17):9463. doi: 10.3390/ijms25179463. Int J Mol Sci. 2024. PMID: 39273409 Free PMC article. Review.
References
-
- Mueller H. The remaking of chromosomes. Collecting Net Woods Hole. 1938;13:181–198.
Publication types
LinkOut - more resources
Full Text Sources

