Sinopharm (HB02)-associated vaccine-induced immune thrombotic thrombocytopenia: a case report
- PMID: 37679815
- PMCID: PMC10486117
- DOI: 10.1186/s13256-023-04086-7
Sinopharm (HB02)-associated vaccine-induced immune thrombotic thrombocytopenia: a case report
Abstract
Background: Vaccine-induced thrombotic thrombocytopenia is associated with the coronavirus disease 2019 vaccines. It has been reported by vector-based vaccines. To the best of our knowledge, there is no report about vaccine-induced thrombotic thrombocytopenia in whole-virus vaccines. We are presenting the first case of vaccine-induced thrombotic thrombocytopenia with this type of vaccine.
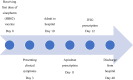
Case presentation: An 18-year-old male Caucasian patient with complaints of severe abdominal, low back, and lower extremity pain presented to the medical center. He received the first dose of the Sinopharm (HB02) vaccine against coronavirus disease 2019 10 days before hospital attendance. In the laboratory examination, decreased platelet count and increased D-dimer were observed. During hospital admission, the diagnosis of pulmonary embolism was reached. He received vaccine-induced thrombotic thrombocytopenia therapy consisting of intravenous immune globulin and direct oral anticoagulant. Platelet count increased and he was discharged after 1 month.
Conclusion: This case highlights the possibility of vaccine-induced thrombotic thrombocytopenia occurrence by whole-virus coronavirus disease 2019 vaccines. Compared with vector-based vaccines, this phenomenon is rare for whole-virus vaccines. More studies on this type of vaccine regarding thrombotic thrombocytopenia should be considered.
Keywords: COVID-19; Case report; Thrombosis; Vaccine; Whole-virus vaccine.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declared none.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical