Efficacy and safety of tart cherry supplementary citrate mixture on gout patients: a prospective, randomized, controlled study
- PMID: 37679816
- PMCID: PMC10483724
- DOI: 10.1186/s13075-023-03152-1
Efficacy and safety of tart cherry supplementary citrate mixture on gout patients: a prospective, randomized, controlled study
Abstract
Background: Low urine pH, which may be mediated by metabolic syndrome (MetS), is common in gout. Tart cherries are shown to improve MetS symptoms and possess anti-inflammatory properties. However, the efficacy of tart cherry supplements on urine pH has yet to be studied.
Objectives: This study aimed to investigate the efficacy and safety of tart cherry supplementary citrate (TaCCi) mixture on urine pH, serum urate (sUA), C-reactive protein (CRP), and gout flares in gout patients initiating urate-lowering therapy (ULT), in comparison to citrate mixture and sodium bicarbonate.
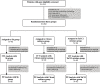
Methods: A prospective, randomized (1:1:1), open-label, parallel-controlled trial was conducted among 282 men with gout and fasting urine pH ≤ 6, who were initiating ULT with febuxostat (initially 20 mg daily, escalating to 40 mg daily if serum urate ≥ 360 μmol/L). Participants were randomized to groups taking either sodium bicarbonate, citrate mixture, or TaCCi mixture. All participants were followed every 4 weeks until week 12. Urine pH and sUA were co-primary outcomes, with various biochemical and clinical secondary endpoints.
Results: Urine pH increased to a similar extent in all three groups. SUA levels declined in all three groups as well, with no significant differences observed between the groups. At week 12, the TaCCi mixture group exhibited a greater reduction in the urine albumin/creatinine ratio (UACR) compared to the other two groups (p < 0.05). Participants taking TaCCi mixture or citrate mixture experienced fewer gout flares than those in the sodium bicarbonate group over the study period (p < 0.05). Additionally, the TaCCi mixture group had a lower CRP level at week 12 relative to the other two groups (p < 0.01). Adverse events were similar across all three groups.
Conclusion: The TaCCi mixture had similar efficacy and safety on urine alkalization and sUA-lowering as the citrate mixture and sodium bicarbonate in patients with gout. However, the TaCCi mixture resulted in greater improvements in UACR and CRP, which suggests that tart cherry supplements may provide additional benefits for renal protection and reduce inflammation in gout, particularly when starting ULT.
Trial registration: This project was registered in ChiCTR ( www.chictr.org.cn ), with the registration number: ChiCTR2100050749.
Keywords: Citrate; Gout; Sodium bicarbonate; Tart cherry; Urine alkalization.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests. Nicola Dalbeth has received consulting fees, speaker fees, or grants from AstraZeneca, Dyve Biosciences, Horizon, Amgen, Selecta, Arthrosi, JW Pharmaceutical Corporation, PK Med, PTC Therapeutics, Protalix, Cello Health, Abbvie, and Janssen, outside the submitted work.
Other authors have nothing to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

