Guilingji capsule for Alzheimer's disease: secondary analysis of a randomized non-inferiority controlled trial
- PMID: 37679990
- PMCID: PMC10465832
- DOI: 10.19852/j.cnki.jtcm.20230404.006
Guilingji capsule for Alzheimer's disease: secondary analysis of a randomized non-inferiority controlled trial
Abstract
Objective: To investigate the effectiveness and safety of Guilingji capsule (, GLJC) in treatment of Alzheimer's disease (AD) patients with kidney-marrow deficiency pattern (KMDP) compared with gingko extract tablets.
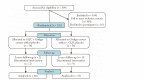
Methods: This is a secondary analysis of a large-scale multicenter randomized non-inferiority clinical trial. A total of 120 AD patients with KMDP were enrolled in this study. The participants were randomly categorized into two groups: (a) GLJC group ( = 60) and (b) gingko group ( = 60). The GLJC group was treated with GLJC and gingko extract mimetic tablets, whereas the gingko group received gingko extract tablets and mimetic GLJC. The data on the Mini-Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), Activities of Daily Living (ADL), and Chinese Medicine Symptom Scale (CM-SS) was evaluated at 0, 12, and 24 weeks of treatment. The serum levels of acetylcholine (Ach), acetylcholinesterase (AchE), B-cell lymphoma-2 (Bcl-2), and Bcl-2-associated X protein (Bax) in the participants were measured before and after 24 weeks of treatment. The safety was based on the incidence of adverse events.
Results: Both interventions significantly increased the MMSE scores of the participants and decreased their ADAS-Cog, ADL, and CM-SS scores ( < 0.01). Compared with the gingko group, the GLJC group had a higher effective rate of improvement in the symptoms of "amnesia" and "dull expression and slow thinking" at the 12th week and 24th week ( < 0.05, < 0.01). In the GLJC group, serum Bcl-2 levels were significantly increased at the 24th week ( < 0.05). Serum Bax and AchE levels of the two groups were significantly decreased at the 24th week ( < 0.01). No treatment-related adverse events were reported in the two groups.
Conclusions: GLJC is equivalent to the gingko extract tablets in terms of improving cognitive function and the quality of life in AD patients with KMDP and has good clinical efficacy and safety. When it comes to improving TCM symptoms and anti-aging, GLJC is even more advantageous.
Keywords: Alzheimer disease; Guilingji capsule; gingko extract tablets; kidney-marrow deficiency pattern; randomized controlled trial.
Figures
References
-
- Alzheimer's Association Report . 2022 Alzheimer's disease facts and figures. Alzheimers Dement 2022; 18: 700-89. - PubMed
-
- Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 2020; 5: e661-71. - PubMed
-
- Zhao SJ, Liu XJ, Tian JS, et al. Effects of Guilingji on aging rats and its underlying mechanisms. Rejuvenation Res 2020; 23: 138-49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials