Lobaplatin induces apoptosis in T24 and 5637 bladder cancer cells by regulating Bcl-2 and Bax expression and inhibiting the PI3K/Akt signaling pathway
- PMID: 37680227
- PMCID: PMC10481196
- DOI: 10.21037/tau-23-376
Lobaplatin induces apoptosis in T24 and 5637 bladder cancer cells by regulating Bcl-2 and Bax expression and inhibiting the PI3K/Akt signaling pathway
Abstract
Background: Lobaplatin (LBP) is a third-generation platinum-based drug that has been approved only in China for the treatment of several cancer types. Nonetheless, its efficacy in treating bladder cancer (BC) is unclear thus far. Through in vitro and in vivo experiments, this study aimed to explore whether LBP has an antitumor effect on T24 and 5637 BC cells and whether the effect is related to B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax) and regulation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway.
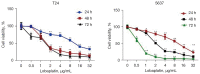
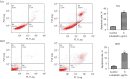
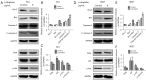
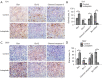
Methods: For in vitro experiments, the cell counting kit-8 (CCK-8) method was used to determine how different concentrations of LBP affect the viability of two types of BC cells. A wound healing assay was used to test the inhibitory effect of LBP on the migration of the two cell lines. Annexin V-fluorescein isothiocyanate isomer I (V-FITC)/propidium iodide (PI) staining was used to detect changes in cell apoptosis before and after LBP treatment, and Western blotting was used to detect the expression of apoptosis-related proteins and PI3K/Akt pathway proteins. For in vivo experiments, a cell-derived xenograft (CDX) model was employed, and the weight of nude mice and the tumor size were measured. Immunohistochemistry was used to detect the effect of LBP on the expression of apoptosis-related proteins in tumor xenografts.
Results: In vitro, LBP reduced proliferation (P<0.05), inhibited migration (P<0.05), and induced apoptosis in T24 (31.25%±1.20%, P<0.01) and 5637 (14.3%±2.24%, P<0.05) BC cells, in a dose-dependent manner (P<0.05); increased the expression of proapoptotic proteins, including Bax, caspase-3 and cleaved caspase-3 (P<0.05); and suppressed the expression of antiapoptotic proteins, including Bcl-2, PI3K, Akt and phosphorylated Akt (p-Akt). The in vivo experiment confirmed that LBP can reduce the size of subcutaneous tumors in nude mice (P<0.05), increase the expression levels of Bax and cleaved caspase-3 and lower the expression of Bcl-2 (P<0.05) in bladder tumor tissue.
Conclusions: The results obtained from both experiments suggest that LBP can inhibit the proliferation of T24 and 5637 BC cells, which might be credited to its effects in regulating Bcl-2 and Bax expression and inhibiting the PI3K/Akt pathway.
Keywords: Lobaplatin (LBP); apoptosis; bladder cancer (BC); phosphoinositide 3-kinase/protein kinase B pathway (PI3K/Akt pathway).
2023 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-376/coif). The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Lycium barbarum polysaccharide reverses drug resistance in oxaliplatin-resistant colon cancer cells by inhibiting PI3K/AKT-dependent phosphomannose isomerase.Front Pharmacol. 2024 Mar 21;15:1367747. doi: 10.3389/fphar.2024.1367747. eCollection 2024. Front Pharmacol. 2024. PMID: 38576495 Free PMC article.
-
Xihuang pills induce apoptosis in hepatocellular carcinoma by suppressing phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin pathway.World J Gastrointest Oncol. 2022 Apr 15;14(4):872-886. doi: 10.4251/wjgo.v14.i4.872. World J Gastrointest Oncol. 2022. PMID: 35582102 Free PMC article.
-
Metformin exerts an antitumor effect by inhibiting bladder cancer cell migration and growth, and promoting apoptosis through the PI3K/AKT/mTOR pathway.BMC Urol. 2022 May 24;22(1):79. doi: 10.1186/s12894-022-01027-2. BMC Urol. 2022. PMID: 35610639 Free PMC article.
-
Paeonol inhibits proliferation and induces cell apoptosis of human T24 and 5637 bladder cancer cells in vitro and in vivo.Clin Transl Oncol. 2021 Mar;23(3):601-611. doi: 10.1007/s12094-020-02455-y. Epub 2020 Jul 20. Clin Transl Oncol. 2021. PMID: 32691366
-
Xanthoceraside induces cell apoptosis through downregulation of the PI3K/Akt/Bcl-2/Bax signaling pathway in cell lines of human bladder cancer.Indian J Pathol Microbiol. 2021 Apr-Jun;64(2):294-301. doi: 10.4103/IJPM.IJPM_462_19. Indian J Pathol Microbiol. 2021. PMID: 33851623
Cited by
-
What is the Reason That the Pharmacological Future of Chemotherapeutics in the Treatment of Lung Cancer Could Be Most Closely Related to Nanostructures? Platinum Drugs in Therapy of Non-Small and Small Cell Lung Cancer and Their Unexpected, Possible Interactions. The Review.Int J Nanomedicine. 2024 Sep 14;19:9503-9547. doi: 10.2147/IJN.S469217. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39296940 Free PMC article. Review.
-
Metal-based molecules in the treatment of cancer: From bench to bedside.Oncol Res. 2025 Mar 19;33(4):759-779. doi: 10.32604/or.2024.057019. eCollection 2025. Oncol Res. 2025. PMID: 40191719 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
