Magnetic resonance imaging in COVID-19-associated acute invasive fungal rhinosinusitis - Diagnosis and beyond
- PMID: 37680251
- PMCID: PMC10481822
- DOI: 10.25259/JCIS_46_2023
Magnetic resonance imaging in COVID-19-associated acute invasive fungal rhinosinusitis - Diagnosis and beyond
Abstract
Objectives: The aim of the study was to evaluate the magnetic resonance imaging (MRI) features of acute invasive fungal rhinosinusitis (AIFRS) at presentation and on follow-up imaging when patients receive treatment with systemic antifungal therapy and surgical debridement.
Material and methods: This is a retrospective analysis of imaging data from a cohort of patients diagnosed with AIFRS during the second wave of COVID-19 in single tertiary referral hospital in South India between March 2021 and May 2021 (n = 68). Final diagnosis was made using a composite reference standard which included a combination of MRI findings, clinical presentation, nasal endoscopy and intraoperative findings, and laboratory proof of invasive fungal infection. Analysis included 62 patients with "Definite AIFRS" findings on MRI and another six patients with "Possible AIFRS" findings on MRI and laboratory proof of invasive fungal infection. Follow-up imaging was available in 41 patients.
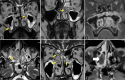
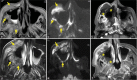
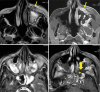
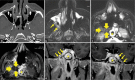
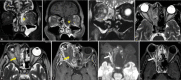
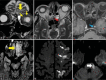
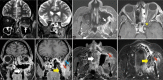
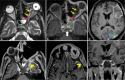
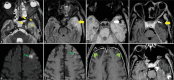
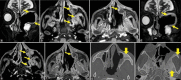
Results: The most frequent MRI finding was T2 hypointensity in the sinonasal mucosa (94%) followed by mucosal necrosis/loss of contrast-enhancement (92.6%). Extrasinosal inflammation with or without necrosis in the pre-antral fat, retroantral fat, pterygopalatine fossa, and masticator space was seen in 91.1% of the cases. Extrasinosal spread was identified on MRI even when the computed tomography (CT) showed intact bone with normal extrasinosal density. Orbital involvement (72%) was in the form of contiguous spread from either the ethmoid or maxillary sinuses; the most frequent presentation being orbital cellulitis and necrosis, with some cases showing extension to the orbital apex (41%) and inflammation of the optic nerve (32%). A total of 22 patients showed involvement of the cavernous sinuses out of which 10 had sinus thrombosis and five patients had cavernous internal carotid artery involvement. Intracranial extension was seen both in the form of contiguous spread to the pachymeninges over the frontal and temporal lobes (25%) and intra-axial involvement in the form of cerebritis, abscesses, and infarcts (8.8%). Areas of blooming on SWI were noted within the areas of cerebritis and infarcts. Perineural spread of inflammation was seen along the mandibular nerves across foramen ovale in five patients and from the cisternal segment of trigeminal nerve to the root exit zone in pons in three patients. During follow-up, patients with disease progression showed involvement of the bones of skull base, osteomyelitis of the palate, alveolar process of maxilla, and zygoma. Persistent hyperenhancement in the post-operative bed after surgical debridement and resection was noted even in patients with stable disease.
Conclusion: Contrast-enhanced MRI must be performed in all patients with suspected AIFRS as non-contrast MRI fails to demonstrate tissue necrosis and CT fails to demonstrate extrasinosal disease across intact bony walls. Orbital apex, pterygopalatine fossa, and the cavernous sinuses form important pathways for disease spread to the skull base and intracranial compartment. While cerebritis, intracranial abscesses, and infarcts can be seen early in the disease due to the angioinvasive nature, perineural spread and skull base infiltration are seen 3-4 weeks after disease onset. Exaggerated soft-tissue enhancement in the post-operative bed after debridement can be a normal finding and must not be interpreted as disease progression.
Keywords: COVID-19; Fungal sinusitis; MRI; Mucormycosis; Rhinosinusitis.
© 2023 Published by Scientific Scholar on behalf of Journal of Clinical Imaging Science.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Skull Base Involvement in Covid Associated Rhino-Orbital-Cerebral Mucormycosis: A Comprehensive Analysis.Indian J Otolaryngol Head Neck Surg. 2023 Apr 8;75(3):1-13. doi: 10.1007/s12070-023-03717-1. Online ahead of print. Indian J Otolaryngol Head Neck Surg. 2023. PMID: 37362115 Free PMC article.
-
Imaging findings using a combined MRI/CT protocol to identify the "entire iceberg" in post-COVID-19 mucormycosis presenting clinically as only "the tip".Clin Radiol. 2021 Oct;76(10):784.e27-784.e33. doi: 10.1016/j.crad.2021.07.002. Epub 2021 Aug 2. Clin Radiol. 2021. PMID: 34353524
-
[Managements and prognostic analyses in patients with invasive fungal rhinosinusitis].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016 Aug 7;51(8):568-72. doi: 10.3760/cma.j.issn.1673-0860.2016.08.002. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016. PMID: 27625124 Chinese.
-
Imaging Features of Invasive Fungal Rhinosinusitis: A Systematic Review.Can Assoc Radiol J. 2024 Aug;75(3):601-608. doi: 10.1177/08465371241227424. Epub 2024 Feb 12. Can Assoc Radiol J. 2024. PMID: 38344986
-
Magnetic Resonance Imaging in Coronavirus Disease - 2019 Associated Rhino-Orbital-Cerebral Mucormycosis (CA-ROCM) - Imaging Analysis of 50 Consecutive Patients.Curr Probl Diagn Radiol. 2022 Jan-Feb;51(1):112-120. doi: 10.1067/j.cpradiol.2021.09.004. Epub 2021 Nov 3. Curr Probl Diagn Radiol. 2022. PMID: 34802841 Free PMC article. Review.
Cited by
-
The Relevance and Potential Role of Orbital Fat in Inflammatory Orbital Diseases: Implications for Diagnosis and Treatment.Ophthalmol Ther. 2025 Feb;14(2):247-281. doi: 10.1007/s40123-024-01079-7. Epub 2024 Dec 16. Ophthalmol Ther. 2025. PMID: 39680323 Free PMC article. Review.
References
-
- Khattar VS, Hathiram BT, Khattar VS, Hathiram BT. Radiologic appearances in fungal rhinosinusitis. Otorhinolaryngol Clin Int J. 2009;1:15–23. doi: 10.5005/jp-journals-10003-1002. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
