3D-printed wound dressings containing a fosmidomycin-derivative prevent Acinetobacter baumannii biofilm formation
- PMID: 37680458
- PMCID: PMC10480667
- DOI: 10.1016/j.isci.2023.107557
3D-printed wound dressings containing a fosmidomycin-derivative prevent Acinetobacter baumannii biofilm formation
Abstract
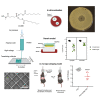
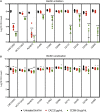
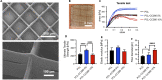
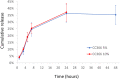
Acinetobacter baumannii causes a wide range of infections, including wound infections. Multidrug-resistant A. baumannii is a major healthcare concern and the development of novel treatments against these infections is needed. Fosmidomycin is a repurposed antimalarial drug targeting the non-mevalonate pathway, and several derivatives show activity toward A. baumannii. We evaluated the antimicrobial activity of CC366, a fosmidomycin prodrug, against a collection of A. baumannii strains, using various in vitro and in vivo models; emphasis was placed on the evaluation of its anti-biofilm activity. We also developed a 3D-printed wound dressing containing CC366, using melt electrowriting technology. Minimal inhibitory concentrations of CC366 ranged from 1 to 64 μg/mL, and CC366 showed good biofilm inhibitory and moderate biofilm eradicating activity in vitro. CC366 successfully eluted from a 3D-printed dressing, the dressings prevented the formation of A. baumannnii wound biofilms in vitro and reduced A. baumannii infection in an in vivo mouse model.
Keywords: Microbiofilms; Microbiology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Lee C.R., Lee J.H., Park M., Park K.S., Bae I.K., Kim Y.B., Cha C.J., Jeong B.C., Lee S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. - DOI - PMC - PubMed
-
- Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources

