Muscarinic Receptor Stimulation Does Not Inhibit Voltage-dependent Ca2+ Channels in Rat Adrenal Medullary Chromaffin Cells
- PMID: 37680574
- PMCID: PMC10480484
- DOI: 10.1267/ahc.23-00042
Muscarinic Receptor Stimulation Does Not Inhibit Voltage-dependent Ca2+ Channels in Rat Adrenal Medullary Chromaffin Cells
Abstract
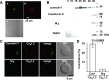
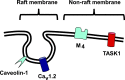
Adrenal medullary chromaffin (AMC) and sympathetic ganglion cells are derived from the neural crest and show a similar developmental path. Thus, these two cell types have many common properties in membrane excitability and signaling. However, AMC cells function as endocrine cells while sympathetic ganglion cells are neurons. In rat sympathetic ganglion cells, muscarinic M1 and M4 receptors mediate excitation and inhibition via suppression of M-type K+ channels and suppression of voltage-dependent Ca2+ channels, respectively. On the other hand, M1 receptor stimulation in rat AMC cells also produces excitation by suppressing TWIK-related acid sensitive K+ (TASK) channels. However, whether M4 receptors are coupled with voltage-dependent Ca2+ channel suppression is unclear. We explore this issue electrophysiologically and biochemically. Electrical stimulation of nerve fibers in rat adrenal glands trans-synaptically increased the Ca2+ signal in AMC cells. This electrically evoked increased Ca2+ signal was not altered during muscarine-induced increase in Ca2+ signal, whereas it decreased significantly during a GABA-induced increase, due to a shunt effect of increased Cl- conductance. The whole-cell current recordings revealed that voltage-dependent Ca2+ currents in AMC cells were suppressed by adenosine triphosphate, but not by muscarinic agonists. The fractionation analysis and immunocytochemistry indicated that CaV1.2 Ca2+ channels and M4 receptors are located in the raft and non-raft membrane domains, respectively. We concluded that muscarinic stimulation in rat AMC cells does not produce voltage-dependent Ca2+ channel inhibition. This lack of muscarinic inhibition is at least partly due to physical separation of voltage-dependent Ca2+ channels and M4 receptors in the plasma membrane.
Keywords: ATP; chromaffin cell; muscarinic receptor; raft membrane domain; voltage-dependent Ca2+ channel.
2023 The Japan Society of Histochemistry and Cytochemistry.
Conflict of interest statement
VThe authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Enhancement of muscarinic receptor-mediated excitation in spontaneously hypertensive rat adrenal medullary chromaffin cells.Auton Neurosci. 2023 Sep;248:103108. doi: 10.1016/j.autneu.2023.103108. Epub 2023 Jul 10. Auton Neurosci. 2023. PMID: 37467550
-
Molecular mechanism for muscarinic M1 receptor-mediated endocytosis of TWIK-related acid-sensitive K+ 1 channels in rat adrenal medullary cells.J Physiol. 2017 Nov 15;595(22):6851-6867. doi: 10.1113/JP275039. Epub 2017 Oct 19. J Physiol. 2017. PMID: 28944482 Free PMC article.
-
M4 muscarinic receptor activation modulates calcium channel currents in rat intracardiac neurons.J Neurophysiol. 1997 Oct;78(4):1903-12. doi: 10.1152/jn.1997.78.4.1903. J Neurophysiol. 1997. PMID: 9325359
-
Muscarinic receptors in adrenal chromaffin cells: physiological role and regulation of ion channels.Pflugers Arch. 2018 Jan;470(1):29-38. doi: 10.1007/s00424-017-2047-2. Epub 2017 Jul 31. Pflugers Arch. 2018. PMID: 28762161 Review.
-
Modulation of ion channels by somatostatin and acetylcholine.Prog Neurobiol. 1992;38(2):203-30. doi: 10.1016/0301-0082(92)90040-l. Prog Neurobiol. 1992. PMID: 1372125 Review.
References
-
- Akiyama, T., Yamazaki, T., Mori, H. and Sunagawa, K. (2003) Inhibition of cholinesterase elicits muscarinic receptor-mediated synaptic transmission in the rat adrenal medulla. Auton. Neurosci. 107; 65–73. - PubMed
-
- Alamo, L., Garcìa, A. G. and Borges, R. (1991) Electrically-evoked catecholamine release from cat adrenals. Role of cholinergic receptors. Biochem. Pharmacol. 42; 973–978. - PubMed
-
- Alicia, S., Angélica, Z., Carlos, S., Alfonso, S. and Vaca, L. (2008) STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium 44; 479–491. - PubMed
-
- Allen, J. A., Halverson-Tamboli, R. A. and Rasenick, M. M. (2007) Lipid raft microdomains and neurotransmitter signaling. Nat. Rev. Neurosci. 8; 128–140. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

