The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions
- PMID: 37680888
- PMCID: PMC10482240
- DOI: 10.3389/fendo.2023.1218148
The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions
Abstract
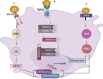
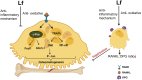
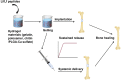
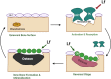
Lactoferrin (Lf) is a multifunctional protein that is synthesized endogenously and has various biological roles including immunological regulation, antibacterial, antiviral, and anticancer properties. Recently, research has uncovered Lf's critical functions in bone remodeling, where it regulates the function of osteoblasts, chondrocytes, osteoclasts, and mesenchymal stem cells. The signaling pathways involved in Lf's signaling in osteoblasts include (low density lipoprotein receptor-related protein - 1 (LRP-1), transforming growth factor β (TGF-β), and insulin-like growth factor - 1 (IGF-1), which activate downstream pathways such as ERK, PI3K/Akt, and NF-κB. These pathways collectively stimulate osteoblast proliferation, differentiation, and mineralization while inhibiting osteoclast differentiation and activity. Additionally, Lf's inhibitory effect on nuclear factor kappa B (NF-κB) suppresses the formation and activity of osteoclasts directly. Lf also promotes chondroprogenitor proliferation and differentiation to chondrocytes by activating the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphoinositide 3-kinase/protein kinase B(PI3K/Akt)signaling pathways while inhibiting the expression of matrix-degrading enzymes through the suppression of the NF-κB pathway. Lf's ability to stimulate osteoblast and chondrocyte activity and inhibit osteoclast function accelerates fracture repair, as demonstrated by its effectiveness in animal models of critical-sized long bone defects. Moreover, studies have indicated that Lf can rescue dysregulated bone remodeling in osteoporotic conditions by stimulating bone formation and suppressing bone resorption. These beneficial effects of Lf on bone health have led to its exploration in nutraceutical and pharmaceutical applications. However, due to the large size of Lf, small bioactive peptides are preferred for pharmaceutical applications. These peptides have been shown to promote bone fracture repair and reverse osteoporosis in animal studies, indicating their potential as therapeutic agents for bone-related diseases. Nonetheless, the active concentration of Lf in serum may not be sufficient at the site requiring bone regeneration, necessitating the development of various delivery strategies to enhance Lf's bioavailability and target its active concentration to the site requiring bone regeneration. This review provides a critical discussion of the issues mentioned above, providing insight into the roles of Lf in bone remodeling and the potential use of Lf as a therapeutic target for bone disorders.
Keywords: bone remodeling; fracture repair; lactoferrin; osteoporosis; signaling pathways.
Copyright © 2023 Tian, Han, Yang, Li, Shi and Tian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Lactoferrin stimulates osteoblast differentiation through PKA and p38 pathways independent of lactoferrin's receptor LRP1.J Bone Miner Res. 2014;29(5):1232-43. doi: 10.1002/jbmr.2116. J Bone Miner Res. 2014. PMID: 24877241
-
Activation of TGF-β Canonical and Noncanonical Signaling in Bovine Lactoferrin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells.Int J Mol Sci. 2019 Jun 13;20(12):2880. doi: 10.3390/ijms20122880. Int J Mol Sci. 2019. PMID: 31200471 Free PMC article.
-
Lactoferrin induces tropoelastin expression by activating the lipoprotein receptor-related protein 1-mediated phosphatidylinositol 3-kinase/Akt pathway in human dermal fibroblasts.Cell Biol Int. 2017 Dec;41(12):1325-1334. doi: 10.1002/cbin.10845. Epub 2017 Sep 8. Cell Biol Int. 2017. PMID: 28833753
-
Lactoferrin in Bone Tissue Regeneration.Curr Med Chem. 2020;27(6):838-853. doi: 10.2174/0929867326666190503121546. Curr Med Chem. 2020. PMID: 31258057 Review.
-
Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns.Int J Biochem Cell Biol. 2013 Aug;45(8):1730-47. doi: 10.1016/j.biocel.2013.04.028. Epub 2013 May 6. Int J Biochem Cell Biol. 2013. PMID: 23660296 Review.
Cited by
-
MAD-microbial (origin of) Alzheimer's disease hypothesis: from infection and the antimicrobial response to disruption of key copper-based systems.Front Neurosci. 2024 Oct 2;18:1467333. doi: 10.3389/fnins.2024.1467333. eCollection 2024. Front Neurosci. 2024. PMID: 39416952 Free PMC article. Review.
-
Addressing osteoblast senescence: Molecular pathways and the frontier of anti-ageing treatments.Clin Transl Med. 2025 Jul;15(7):e70417. doi: 10.1002/ctm2.70417. Clin Transl Med. 2025. PMID: 40681473 Free PMC article. Review.
-
Topical oxygen therapy as a novel strategy to promote wound healing and control the bacteria in implantology, oral surgery and periodontology: A review.Saudi Dent J. 2024 Jun;36(6):841-854. doi: 10.1016/j.sdentj.2024.04.004. Epub 2024 Apr 19. Saudi Dent J. 2024. PMID: 38883907 Free PMC article. Review.
-
The Promotion of Cell Proliferation by Food-Derived Bioactive Peptides: Sources and Mechanisms.Metabolites. 2025 Jul 29;15(8):505. doi: 10.3390/metabo15080505. Metabolites. 2025. PMID: 40863124 Free PMC article. Review.
-
NOR1 promotes the osteoblastic differentiation of human periodontal ligament stem cells via TGF-β signaling pathway.Cell Mol Life Sci. 2024 Aug 9;81(1):338. doi: 10.1007/s00018-024-05356-3. Cell Mol Life Sci. 2024. PMID: 39120703 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

