Ixazomib, daratumumab and low-dose dexamethasone in intermediate-fit patients with newly diagnosed multiple myeloma: an open-label phase 2 trial
- PMID: 37680948
- PMCID: PMC10481174
- DOI: 10.1016/j.eclinm.2023.102167
Ixazomib, daratumumab and low-dose dexamethasone in intermediate-fit patients with newly diagnosed multiple myeloma: an open-label phase 2 trial
Abstract
Background: The outcome of non-transplant eligible newly diagnosed multiple myeloma (NDMM) patients is heterogeneous, partly depending on frailty level. The aim of this study was to prospectively investigate the efficacy and safety of Ixazomib-Daratumumab-low-dose dexamethasone (Ixa-Dara-dex) in NDMM intermediate-fit patients.
Methods: In this phase II multicenter HOVON-143 study, IMWG Frailty index based intermediate-fit patients, were treated with 9 induction cycles of Ixa-Dara-dex, followed by maintenance with ID for a maximum of 2 years. The primary endpoint was overall response rate on induction treatment. Patients were included from October 2017 until May 2019. Trial Registration Number: NTR6297.
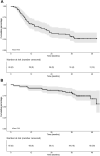
Findings: Sixty-five patients were included. Induction therapy resulted in an overall response rate of 71%. Early mortality was 1.5%. At a median follow-up of 41.0 months, median progression-free survival (PFS) was 18.2 months and 3-year overall survival 83%. Discontinuation of therapy occurred in 77% of patients, 49% due to progression, 9% due to toxicity, 8% due to incompliance, 3% due to sudden death and 8% due to other reasons. Dose modifications of ixazomib were required frequently (37% and 53% of patients during induction and maintenance, respectively), mainly due to, often low grade, polyneuropathy. During maintenance 23% of patients received daratumumab alone. Global quality of life (QoL) improved significantly and was clinically relevant, which persisted during maintenance treatment.
Interpretation: Ixazomib-Daratumumab-low-dose dexamethasone as first line treatment in intermediate-fit NDMM patients is safe and improves global QoL. However, efficacy was limited, partly explained by ixazomib-induced toxicity, hampering long term tolerability of this 3-drug regimen. This highlights the need for more efficacious and tolerable regimens improving the outcome in vulnerable intermediate-fit patients.
Funding: Janssen Pharmaceuticals, Takeda Pharmaceutical Company Limited.
Keywords: Daratumumab; Elderly; IMWG frailty index; Intermediate-fit; Ixazomib; Multiple myeloma.
© 2023 The Author(s).
Conflict of interest statement
Claudia Stege. Speaker's Bureau: Sanofi, Celgene/Bristol Myers Squibb, Takeda. Consulting or Advisory Role: Sanofi, Janssen. Marie-Christiane Vekemans. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Amgen, Janssen, Takeda, Bristol Myers Squibb/Celgene. Consulting or Advisory Role: Amgen, Celgene-Bristol Myers Squibb, Janssen, Takeda, Sanofi, Pfizer, GlaxoSmithKline, Menarini. Ka-Lung Wu. Consulting or Advisory Role: Pfizer, Janssen, Bristol Myers Squibb. Niels W. C. J. van de Donk. Consulting or Advisory Role: Janssen, Celgene, Bristol Myers Squibb, Novartis, Amgen, Servier, Takeda, Bayer, Roche, Pfizer, Abbvie, Adaptive. Research Funding: Janssen, Celgene, Amgen, Novartis, Bristol Myers Squibb, Cellectis. Gert Jan Timmers. Participation on an Advisory Board: Novartis. Travel, Accommodations, Expenses: Novartis, Janssen. Ellen van der Spek. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Janssen. Pieter Sonneveld. Participation on a Data Safety Monitoring Board or Advisory Board: Celgene, Janssen, Amgen, Bristol Myers Squibb, Karyopharm Therapeutics, Pfizer. Research Funding: Janssen, Amgen, Bristol Myeres Squibb/Celgene, Karyopharm Therapeutics, Pfizer. Inger S. Nijhof. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Janssen, Celgene/Bristol Myers Squibb, Sanofi. Mark-David Levin. Support for attending meetings and/or travel: Janssen, Takeda. Paula F. Ypma. Payment or honoraria for presentations: Janssen. Support for attending meetings and/or travel: Janssen. Sonja Zweegman. Consulting or Advisory Role: Janssen-Cilag, Takeda, Celgene/Bristol Myers Squibb, Sanofi, Oncopeptides (no personal funding). Research Funding: Janssen, Takeda. No other potential conflicts of interest were reported.
Figures

References
LinkOut - more resources
Full Text Sources

