Biophysical tools to study the oligomerization dynamics of Prx1-class peroxiredoxins
- PMID: 37681093
- PMCID: PMC10480382
- DOI: 10.1007/s12551-023-01076-3
Biophysical tools to study the oligomerization dynamics of Prx1-class peroxiredoxins
Abstract
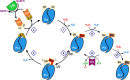
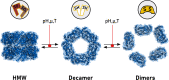
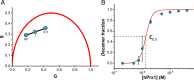
Peroxiredoxins (Prx) are ubiquitous, highly conserved peroxidases whose activity depends on catalytic cysteine residues. The Prx1-class of the peroxiredoxin family, also called typical 2-Cys Prx, organize as head-to-tail homodimers containing two active sites. The peroxidatic cysteine CP of one monomer reacts with the peroxide substrate to form sulfenic acid that reacts with the resolving cysteine (CR) of the adjacent subunit to form an intermolecular disulfide, that is reduced back by the thioredoxin/thioredoxin reductase/NADPH system. Although the minimal catalytic unit is the dimer, these Prx oligomerize into (do)decamers. In addition, these ring-shaped decamers can pile-up into high molecular weight structures. Prx not only display peroxidase activity reducing H2O2, peroxynitrous acid and lipid hydroperoxides (antioxidant enzymes), but also exhibit holdase activity protecting other proteins from unfolding (molecular chaperones). Highly relevant is their participation in redox cellular signaling that is currently under active investigation. The different activities attributed to Prx are strongly ligated to their quaternary structure. In this review, we will describe different biophysical approaches used to characterize the oligomerization dynamics of Prx that include the classical size-exclusion chromatography, analytical ultracentrifugation, calorimetry, and also fluorescence anisotropy and lifetime measurements, as well as mass photometry.
Keywords: AUC; Anisotropy; Lifetime fluorescence; Oligomerization; Peroxiredoxins; Phasors; Quaternary structure; SEC.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures

References
-
- Cao Z, Tavender TJ, Roszak AW, Cogdell RJ, Bulleid NJ. Crystal structure of reduced and of oxidized peroxiredoxin IV enzyme reveals a stable oxidized decamer and a non-disulfide-bonded intermediate in the catalytic cycle. J Biol Chem. 2011;286(49):42257–42266. doi: 10.1074/jbc.M111.298810. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

