Platinum-based chemotherapy for early triple-negative breast cancer
- PMID: 37681577
- PMCID: PMC10486188
- DOI: 10.1002/14651858.CD014805.pub2
Platinum-based chemotherapy for early triple-negative breast cancer
Abstract
Background: Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer associated with shorter survival and a higher likelihood of the cancer returning. In early TNBC, platinum-based chemotherapy has been shown to improve pathological complete response (pCR); however, its effect on long-term survival outcomes has not been fully elucidated and recommendations to include platinum chemotherapy are not consistent in international guidelines.
Objectives: To evaluate the benefits and harms of platinum-based chemotherapy as adjuvant and neoadjuvant treatment in people with early triple-negative breast cancer.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was 4 April 2022.
Selection criteria: We included randomised controlled trials examining neoadjuvant or adjuvant platinum chemotherapy for early TNBC.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were disease-free survival (DFS) and overall survival (OS). Our secondary outcomes were pCR, treatment adherence, grade III or IV toxicity related to chemotherapy, and quality of life. Prespecified subgroups included BRCA mutation status, homologous recombination deficiency (HRD) status, frequency of chemotherapy, type of platinum agent used, and the presence or absence of anthracycline chemotherapy. We assessed risk of bias using Cochrane's RoB 1 tool and certainty of evidence using the GRADE approach.
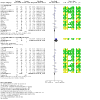
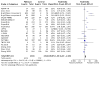
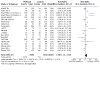
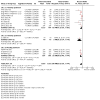
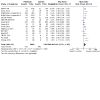
Main results: From 3972 records, we included 20 published studies involving 21 treatment comparisons, and 25 ongoing studies. For most domains, risk of bias was low across studies. There were 16 neoadjuvant chemotherapy studies (one of which combined neoadjuvant and adjuvant therapy) and four adjuvant chemotherapy trials. Most studies used carboplatin (17 studies) followed by cisplatin (two), and lobaplatin (one). Eight studies had an anthracycline-free intervention arm, five of which had a carboplatin-taxane intervention compared to an anthracycline-taxane control. All studies reporting DFS and OS used carboplatin. Inclusion of platinum chemotherapy improved DFS in neoadjuvant and adjuvant settings (neoadjuvant: hazard ratio (HR) 0.63, 95% confidence interval (CI) 0.53 to 0.75; 7 studies, 8 treatment comparisons, 1966 participants; high-certainty evidence; adjuvant: HR 0.69, 95% CI 0.54 to 0.88; 4 studies, 1256 participants; high-certainty evidence). Platinum chemotherapy in the regimen improved OS (neoadjuvant: HR 0.69, 95% CI 0.55 to 0.86; 7 studies, 8 treatment comparisons, 1973 participants; high-certainty evidence; adjuvant: 0.70, 95% CI 0.50 to 0.96; 4 studies, 1256 participants; high-certainty evidence). Median follow-up for survival outcomes ranged from 36 to 97.6 months. Our analysis confirmed platinum chemotherapy increased pCR rates (risk ratio (RR) 1.44, 95% CI 1.31 to 1.59; 15 studies, 16 treatment comparisons, 3083 participants; high-certainty evidence). Subgroup analyses showed no evidence of differences in DFS according to BRCA mutation status, HRD status, lymph node status, or whether the intervention arm contained anthracycline chemotherapy or not. Platinum chemotherapy was associated with reduced dose intensity, with participants more likely to require chemotherapy delays (RR 2.23, 95% CI 1.70 to 2.94; 4 studies, 5 treatment comparisons, 1053 participants; moderate-certainty evidence), dose reductions (RR 1.77, 95% CI 1.56 to 2.02; 7 studies, 8 treatment comparisons, 2055 participants; moderate-certainty evidence) and early cessation of treatment (RR 1.20, 95% CI 1.04 to 1.38; 16 studies, 17 treatment comparisons, 4178 participants; moderate-certainty evidence). Increased haematological toxicity occurred in the platinum group who were more likely to experience grade III/IV neutropenia (RR 1.53, 95% CI 1.43 to 1.63; 19 studies, 20 treatment comparisons, 4849 participants; moderate-certainty evidence), anaemia (RR 8.20, 95% CI 5.66 to 11.89; 18 studies, 19 treatment comparisons, 4757 participants; moderate-certainty evidence) and thrombocytopenia (RR 7.59, 95% CI 5.10 to 11.29; 18 studies, 19 treatment comparisons, 4731 participants; moderate-certainty evidence). There was no evidence of a difference between chemotherapy groups in febrile neutropenia (RR 1.16, 95% CI 0.89 to 1.49; 11 studies, 3771 participants; moderate-certainty evidence). Renal impairment was very rare (0.4%, 2 events in 463 participants; note 3 studies reported 0 events in both arms; 4 studies; high-certainty evidence). Treatment-related death was very rare (0.2%, 7 events in 3176 participants and similar across treatment groups; RR 0.58, 95% 0.14 to 2.33; 10 studies, 11 treatment comparisons; note 8 studies reported treatment-related deaths but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 3 studies rather than 11; 3176 participants; high-certainty evidence). Five studies collected quality of life data but did not report them.
Authors' conclusions: Platinum-based chemotherapy using carboplatin in the adjuvant or neoadjuvant setting improves long-term outcomes of DFS and OS in early TNBC, with no evidence of differences by subgroup. This was at the cost of more frequent chemotherapy delays and dose reductions, and greater haematological toxicity, though serious adverse events including neuropathy, febrile neutropenia or treatment-related death were not increased. These findings support the use of platinum-based chemotherapy for people with early TNBC. The optimal dose and regimen are not defined by this analysis, but there is a suggestion that similar relative benefits result from the addition of carboplatin to either anthracycline-free regimens or those containing anthracycline agents.
Copyright © 2023 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
SM: none.
MW: none. Melina is a member of the Cochrane Breast Cancer editorial team but was not involved in the editorial process for this review.
SE: none.
JB: none related to this review. Jane has received funding to travel to conferences from Novartis. Jane has served on advisory boards for Pfizer and Lilly for unrelated matters and payment has been made to her institution.
RD: none.
AG: none related to this review. Annabel reports consultancy/expert witness or advisory roles for Pfizer (2018, 2019) and AstraZeneca (2018) for matters unrelated to this review topic. There is no competing interest associated with funding of travel to attend an educational meeting, or to provide expertise regarding Cancer Genetic counselling in Australia. Annabel also receives ongoing funds for editorial support for manuscript writing for the EMBRACA trial (not related to this review). Annabel is also a member of the Cochrane Breast Cancer editorial team but was not involved in the editorial process for this review.
Figures











































Update of
- doi: 10.1002/14651858.CD014805
References
References to studies included in this review
ADAPT‐TN {published data only}
-
- Gluz O, Liedtke C, Prat A, Christgen M, Gebauer D, Kates RE, et al. Association of molecular subtype, proliferation, and immune genes with efficacy of carboplatin versus gemcitabine addition to taxane-based, anthracycline-free neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC): results of the randomized WSG ADAPT-TN trial. Journal of Clinical Oncology 2017;35(15 Suppl):573.
-
- Gluz O, Nitz U, Christgen M, Grischke EM, Forstbauer H, Braun MW, et al. Efficacy of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple-negative breast cancer: WSG-ADAPT TN randomized phase II trial. Journal of Clinical Oncology 2015;33(15 Suppl):1032.
-
- Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, et al. Comparison of neoadjuvant nab-paclitaxel+carboplatin vs nab-paclitaxel+gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. Journal of the National Cancer Institute 2018;110(6):628-37. - PubMed
-
- Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, et al. Impact of 12 weeks nab-paclitaxel + carboplatin or gemcitabine followed by anthracycline administration according to pCR in triple-negative early breast cancer: survival results of WSG-ADAPT-TN phase II trial. Journal of Clinical Oncology 2018;36(15 Suppl):573.
-
- Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, et al. Prognostic impact of anthracyclines and immune/proliferation markers in TNBC according to pCR after de-escalated neoadjuvant chemotherapy with 12 weeks of nab-paclitaxel/carboplatin or gemcitabine: Survival results of WSG-ADAPT-TN phase II trial. Annals of Oncology 2018;29(Suppl 8):viii703.
Ando 2014 {published data only}
-
- Ando M, Yamauchi H, Aogi K, Shimizu S, Iwata H, Masuda N, et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Research and Treatment 2014;145(2):401-9. - PubMed
-
- Iwase M, Ando M, Aogi K, Aruga T, Inoue K, Shimomura A, et al. Long-term survival analysis of addition of carboplatin to neoadjuvant chemotherapy in HER2-negative breast cancer. Breast Cancer Research and Treatment 2020;180(3):687-94. - PubMed
-
- Tamura K, Hashimoto J, Tsuda H, Yoshida M, Yamauchi H, Aogi K, et al. Randomized phase II study of weekly paclitaxel with or without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA HER2-negative breast cancer. Journal of Clinical Oncology 2014;32(15 Suppl):1017.
-
- UMIN000003355. Randomized phase II study of preoperative systemic chemotherapy of carboplatin/weekly paclitaxel followed by CEF versus weekly paclitaxel followed by CEF for operable HER2-negative breast cancer. Center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000004074 (first received 22 March 2010).
BrighTNess comparison 1 {published data only}
-
- EudraCT2013-002377-21. A study evaluating safety and efficacy of the addition of ABT-888 plus carboplatin versus the addition of carboplatin to standard chemotherapy versus standard chemotherapy in subjects with early stage triple negative breast cancer. clinicaltrialsregister.eu/ctr-search/search?query=2013-002377-21 (first received 17 November 2014).
-
- Filho OM, Stover DG, Asad S, Ansell PJ, Watson M, Loibl S, et al. Association of immunophenotype with pathologic complete response to neoadjuvant chemotherapy for triple-negative breast cancer: a secondary analysis of the BrighTNess phase 3 randomized clinical trial. JAMA Oncology 2021;7(4):603-8. - PMC - PubMed
-
- Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O'Shaughnessy J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Annals of Oncology 2022;33(4):384-94. - PubMed
-
- Golshan M, Loibl S, Huober JB, O'Shaughnessy J, Rugo HS, Wolmark N, et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from an international randomized trial (BrighTNess). Journal of Clinical Oncology 2017;35(15 Suppl):514.
BrighTNess comparison 2 {published data only}
-
- EudraCT2013-002377-21. A study evaluating safety and efficacy of the addition of ABT-888 plus carboplatin versus the addition of carboplatin to standard chemotherapy versus standard chemotherapy in subjects with early stage triple negative breast cancer. clinicaltrialsregister.eu/ctr-search/search?query=2013-002377-21 (first received 17 November 2014).
-
- Filho OM, Stover DG, Asad S, Ansell PJ, Watson M, Loibl S, et al. Association of immunophenotype with pathologic complete response to neoadjuvant chemotherapy for triple-negative breast cancer: a secondary analysis of the BrighTNess phase 3 randomized clinical trial. JAMA Oncology 2021;7(4):603-8. - PMC - PubMed
-
- Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O'Shaughnessy J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Annals of Oncology 2022;33(4):384-94. - PubMed
-
- Golshan M, Loibl S, Huober JB, O'Shaughnessy J, Rugo HS, Wolmark N, et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from an international randomized trial (BrighTNess). Journal of Clinical Oncology 2017;35(15 Suppl):514.
CALGB 40603 {published data only}
-
- Golshan M, Cirrincione CT, Carey LA, Sikov WM, Berry DA, Burstein HJ, et al. Impact of neoadjuvant therapy on breast conservation rates in triple negative and HER2-positive breast cancer: combined results of CALGB 40603 and 40601 (Alliance). Journal of Clinical Oncology 2015;33(15 Suppl):1007.
-
- Golshan M, Cirrincione CT, Sikov WM, Berry DA, Jasinski S, Weisberg TF, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Annals of Surgery 2015;262(3):434-9. - PMC - PubMed
-
- NCT00861705. Paclitaxel with or without carboplatin and/or bevacizumab followed by doxorubicin and cyclophosphamide in treating patients with breast cancer that can be removed by surgery. clinicaltrials.gov/show/NCT00861705 (first received 13 March 2009).
-
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. - PMC - PubMed
-
- Shepherd JH, Hyslop T, Fan C, Martinez AF, Parker J, Hoadley K, et al. Genomic analysis of the CALGB 40603 (Alliance) neoadjuvant trial in TNBC identifies immune features associated with pathological complete response and event-free survival. Cancer Research 2021;81(4 Suppl):PD9-03.
CALGB 40603 – comparison 1 (without bevacizumab) {published data only}
-
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. - PMC - PubMed
-
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603. Journal of Clinical Oncology 2015;33(1):13-21. - PMC - PubMed
CALGB 40603 – comparison 2 (with bevacizumab) {published data only}
-
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. - PMC - PubMed
-
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603. Journal of Clinical Oncology 2015;33(7):13-21. - PMC - PubMed
GEICAM 2006‐03 {published data only}
-
- Alba E, Chacon JI, Lluch A, Anton A, Estevez L, Cirauqui B, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Research and Treatment 2012;136(2):487-93. - PubMed
-
- Alba E, Chacon JI, Lluch A, Garcia-Estevez L, Anton A, Cirauqui B, et al. Chemotherapy (CT) with or without carboplatin as neoadjuvant treatment in patients with basal-like breast cancer: GEICAM 2006-03 – a multicenter, randomized phase II study. Journal of Clinical Oncology 2011;29(15 Suppl):1015.
-
- NCT00432172. Selective neoadjuvant treatment according to immunohistochemical subtype for HER2 negative breast cancer patients. clinicaltrials.gov/show/NCT00432172 (first received 7 February 2007).
-
- Santonja A, Albanell J, Chacon JI, Lluch A, Sanchez-Munoz A, Rojo F, et al. Triple-negative breast cancer subtypes and pathologic complete-response rate to neoadjuvant chemotherapy: results from the GEICAM/2006-2003 study. Journal of Clinical Oncology 2014;32(15 Suppl):1024.
GeparOcto {published data only}
-
- NCT02125344. A phase III trial comparing two dose-dense, dose-intensified approaches (ETC and PM(Cb)) for neoadjuvant treatment of patients with high-risk early breast cancer (GeparOcto). clinicaltrials.gov/show/NCT02125344. (first received 29 April 2014).
-
- Pohl E, Schneeweiss A, Hauke J, Moebus V, Furlanetto J, Denkert C, et al. Germline mutation status and therapy response in patients with triple negative breast cancer (TNBC): results of the GeparOcto study. Annals of Oncology 2018;29(8 Suppl):viii77.
-
- Schneeweiss A, Mobus V, Tesch H, Hanusch C, Denkert C, Lubbe K, et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): a randomised phase III trial. European Journal of Cancer 2019;106:181-92. - PubMed
-
- Schneeweiss A, Mobus V, Tesch H, Klare P, Denkert C, Kast K, et al. Survival analysis of the randomized phase III GeparOcto trial comparing neoadjuvant chemotherapy (NACT) of iddEPC versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer, TNBC) (PM(Cb)) for patients (pts) with high-risk early breast cancer (BC). Annals of Oncology 2020;31(4 Suppl):S303.
GeparOLA {published data only}
-
- EUDRACT2015-003509-41-DE. A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel / carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients with HER2-negative early breast cancer and homologous recombination deficiency (HRD) (GeparOla). clinicaltrialsregister.eu/ctr-search/search?query=2015-003509-41 (first received 1 August 2016).
-
- Fasching PA, Blohmer JU, Burchardi N, Costa SD, Denkert C, Hanusch C, et al. A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients with HER2-negative early breast cancer and homologous recombination deficiency (HRD): GeparOLA. Journal of Clinical Oncology 2016;34(15 Suppl):TPS1096.
-
- Fasching PA, Jackisch C, Rhiem K, Schneeweiss A, Klare P, Hanusch C, et al. GeparOLA: a randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients (pts) with HER2-negative early breast cancer. Journal of Clinical Oncology 2019;37(15 Suppl):506.
-
- Fasching PA, Link T, Hauke J, Seither F, Jackisch C, Klare P, et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Annals of Oncology 2021;32(1):49-57. - PubMed
-
- Hauke J, Ernst C, Fasching PA, Jackisch C, Seither F, Klare P, et al. Germline mutation status and therapy response in patients with homologous recombination deficient, HER2-negative early breast cancer: results of the GeparOLA study (NCT02789332). Annals of Oncology 2020;31(4 Suppl):S313.
GeparSixto {published data only}
-
- Darb-Esfahani S, Weichert W, Denkert C, Minckwitz G, Nekljudova V, Lindner J, et al. P53 mutations in HER2 positive and triple negative breast cancer treated with neoadjuvant chemotherapy – a translational subproject of the GeparSixto trial. Annals of Oncology 2015;26(Suppl 3):III15.
-
- Denkert C, Loibl S, Salat C, Sinn BV, Schem C, Endris V, et al. Increased tumor-associated lymphocytes predict benefit from addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer in the GeparSixto trial (GBG 66). Cancer Research 2013;73(24 Suppl):S1-06.
-
- Denkert C, Minckwitz G, Brase JC, Darb-Esfahani S, Gade S, Kronenwett R, et al. Expression of immunologic genes in triple-negative and HER2-positive breast cancer in the neoadjuvant GEPARSIXTO trial: prediction of response to carboplatin-based chemotherapy. Journal of Clinical Oncology 2014;32(15 Suppl):510.
-
- Denkert C, Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. Journal of Clinical Oncology 2015;33(9):983-91. - PubMed
-
- EUDRAC2011-000553-23-DE. A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer – GeparSixto. clinicaltrialsregister.eu/ctr-search/search?query=2011-000553-23 (first received 29 September 2011).
Gigolaeva 2019 {published data only}
-
- Gigolaeva L, Krivorotko P, Zhiltsova E, Dashyan G, Chadjimatova S, Pesotckiy R, et al. Neoadjuvant chemotherapy regimens for triple negative breast cancer patients. Breast 2019;44(Suppl 1):S70.
INFORM {published data only}
-
- NCT01670500. Cisplatin vs. doxorubicin/cyclophosphamide in BRCA. clinicaltrials.gov/show/NCT01670500 (first received 22 August 2012).
-
- Tung N, Arun B, Hacker MR, Hofstatter E, Toppmeyer DL, Isakoff SJ, et al. TBCRC 031: randomized phase II study of neoadjuvant cisplatin versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial). Journal of Clinical Oncology 2020;38(14):1539-48. - PMC - PubMed
-
- Tung N, Arun B, Hofstatter E, Hacker MR, Toppmeyer DL, Isakoff SJ, et al. Cisplatin versus doxorubicin/cyclophosphamide as neoadjuvant treatment in germline BRCA mutation carriers (BRCA carriers) with HER2-negative breast cancer: results from the INFORM trial (TBCRC 031). Cancer Research 2019;80(4 Suppl):GS6-03. - PMC - PubMed
I‐SPY2 {published data only}
-
- Liu MC, Symmans WF, Yau C, Chen YY, Rugo HS, Olopade OF, et al. Residual cancer burden (RCB) with veliparib/carboplatin in the I-SPY2 trial. Cancer Research 2016;76(4 Suppl):P3-07-49.
-
- NCT01042379. I-SPY TRIAL: neoadjuvant and personalized adaptive novel agents to treat breast cancer (I-SPY). clinicaltrials.org/show/NCT01042379 (first received 5 January 2010).
-
- Printz C. I-SPY2 trial yields first results on combination therapy for triple-negative breast cancer. Cancer 2014;120(6):773. - PubMed
-
- Van't Veer L, Esserman L, Sanil A, Glas A, Severson T, Linn SC, et al. DNA repair deficiency biomarkers and identification of ER positive breast cancer patients who may benefit from veliparib/carboplatin: results from the I-SPY 2 trial. Journal of Clinical Oncology 2015;33(15 Suppl):521.
Li 2020 {published data only}
-
- Mu Y, Zhang T, Han Y, Wang J, Li Q, Luo Y, et al. A randomized phase III trial comparing dose-dense epirubicin and cyclophosphamide (ECdd) followed by paclitaxel (T) with paclitaxel plus carboplatin (PCdd) as adjuvant chemotherapy for early triple negative breast cancer patients with high recurrence risk. Journal of Clinical Oncology 2019;37(15 Suppl):528.
-
- NCT01378533. The trial comparing dose-dense AC-T with PC as adjuvant therapy for TNBC. clinicaltrials.gov/show/NCT01378533 (first received 22 June 2011).
-
- Wang J, Li Q, Mu Y, Zhang T, Han Y, Wang J, et al. A randomized phase III trial comparing dose-dense epirubicin and cyclophosphamide (ECdd) followed by paclitaxel (T) with paclitaxel plus carboplatin (PCdd) as adjuvant chemotherapy for early triple-negative breast cancer patients with high-recurrence risk. Journal of Clinical Oncology 2019;37(15 Suppl):528.
Nasr 2015 {published data only}
NeoCART {published data only}
-
- NCT03154749. TC (docetaxel/carboplatin) versus EC-T (epirubicin/cyclophosphamide followed by docetaxe) as neoadjuvant chemotherapy for triple-negative breast cancer. clinicaltrials.gov/show/NCT03154749 (first received 16 May 2017).
-
- Zhang L, Wu Z, Lin Y, Li J, Liu Z, Cao Y, et al. Neoadjuvant docetaxel + carboplatin versus epirubicin+cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): results from a multicenter, randomized controlled, open-label, phase II trial. Journal of Clinical Oncology 2022;38(15 Suppl):586. - PubMed
-
- Zhang L, Wu ZY, Li J, Lin Y, Liu Z, Cao Y, et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): results from a multicenter, randomized controlled, open-label phase II trial. International Journal of Cancer 2022;150(4):654-62. - PubMed
PATTERN {published data only}
-
- NCT01216111. Comparison study of adjuvant chemotherapy for Chinese triple negative breast cancer. clinicaltrials.gov/show/NCT01216111 (first received 7 October 2010).
-
- NCT04031703. Study comparing paclitaxel plus carboplatin versus anthracyclines followed by docetaxel as adjuvant chemotherapy for triple negative breast cancer (PATTERN). clinicaltrials.gov/show/NCT04031703 (first received 24 July 2019).
TBCRC 030 {published data only}
-
- Mayer EL, Abramson V, Jankowitz R, Falkson C, Marcom PK, Traina T, et al. TBCRC 030: a phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: evaluating the homologous recombination deficiency (HRD) biomarker. Annals of Oncology 2020;31(11):1518-25. - PMC - PubMed
-
- Mayer EL, Abramson VG, Jankowitz RC, Falkson CI, Marcom PK, Traina TA, et al. TBCRC 030: a randomized phase II study of preoperative cisplatin versus paclitaxel in TNBC- evaluating the homologous recombination deficiency (HRD) biomarker. Journal of Clinical Oncology 2019;37(15 Suppl):507. - PMC - PubMed
-
- Mayer EL, Vaz-Luis I, Richardson AL, Perou CM, Tung NM, Abramson VG, et al. TBCRC030: a randomized, phase II study of preoperative cisplatin versus paclitaxel in patients (pts) with BRCA1/2-proficient triple-negative breast cancer (TNBC) – evaluating the homologous recombination deficiency (HRD) biomarker. Journal of Clinical Oncology 2014;32(15 Suppl):TPS1145.
-
- NCT01982448. Cisplatin vs paclitaxel for triple negative breast cancer. clinicaltrials.gov/show/NCT01982448 (first received 13 November 2013).
Wu 2018 {published data only}
-
- ChiCTR-TRC-14005019. Docetaxel combined with epirubicin and lobaplatin (TEL) in neoadjuvant chemotherapy for triple negative breast cancer: a prospective randomized controlled clinical study. www.chictr.org.cn/showprojEN.html?proj=4555 (first received 29 May 2014).
Zhang 2016 {published data only}
-
- NCT01276769. Comparison study of neoadjuvant paclitaxel plus carboplatin/epirubicin treatment in triple-negative breast cancer. clinicaltrials.gov/show/NCT01276769 (first received 13 January 2011).
-
- Zhang P, Yin Y, Mo H, Zhang B, Wang X, Li Q, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget 2016;7(37):60647-56. - PMC - PubMed
-
- Zhang P, Yin Y, Xu B, Wang X, Zhang B, Li Q, et al. Carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for triple-negative breast cancer – a phase II clinical trial. Cancer Research 2013;73(24 Suppl):P3-14-07.
Zhao 2014 {published data only}
-
- Zhao Y, Li JF, Chu GW. Neoadjuvant chemotherapy regimens for patients with triple-negative breast cancer: TE versus TC. Journal of Practical Oncology 2014;29(6):576-8.
Zheng 2022 {published data only}
-
- Du F, Wang W, Wang Y, Li M, Zhu A, Wang J, et al. Carboplatin plus taxanes are non-inferior to epirubicin plus cyclophosphamide followed by taxanes as adjuvant chemotherapy for early triple-negative breast cancer. Breast Cancer Research and Treatment 2020;182(1):67-77. - PubMed
-
- NCT01150513. Docetaxel+carboplatin vs epirubicin+cyclophosphamide followed by docetaxel as adjuvant treatment in triple-negative breast cancer. clinicaltrials.gov/show/NCT01150513 (first received 25 June 2010).
-
- Yuan P, Du F, Wang J, Ma F, Fan Y, Xu B. Comparison of four cycles epirubicin and cyclophosphamide (EC) followed by four cycles docetaxel (T) versus six cycles docetaxel and carboplatin (TP) as adjuvant chemotherapy for women with operable triple negative breast cancer. Journal of Clinical Oncology 2016;34(Suppl 15):1068.
-
- Yuan P, Xu B. A phase III, randomized trial of docetaxel plus carboplatin (TP) versus epirubicin plus cyclophosphamide followed by docetaxel (EC-T) as adjuvant treatment for triple-negative, early-stage breast cancer in Chinese patients. Journal of Clinical Oncology 2012;30(15 Suppl):TPS1135.
-
- Yuan P, Xu BH, Wang JY, Ma F, Li Q, Zhang P, et al. Docetaxel plus carboplatin versus EC-T as adjuvant chemotherapy for triple-negative breast cancer: safety data from a phase III randomized open-label trial. Chung Hua Chung Liu Tsa Chih 2012;34(6):465-8. - PubMed
References to studies excluded from this review
ECOG‐ACRIN EA1131 {published data only}
-
- Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, et al. A randomized phase III postoperative trial of platinum-based chemotherapy (P) versus capecitabine (C) in patients (pts) with residual triple-negative breast cancer (TNBC) following neoadjuvant chemotherapy (NAC): ECOG-ACRIN EA1131. Journal of Clinical Oncology 2021;39(15 Suppl):605. - PMC - PubMed
-
- Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, et al. Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. Journal of Clinical Oncology 2021;39(23):2539-51. - PMC - PubMed
-
- NCT02445391. Platinum based chemotherapy or capecitabine in treating patients with residual triple-negative basal-like breast cancer following neoadjuvant chemotherapy. clinicaltrials.gov/show/NCT02445391 (first received 15 May 2015).
Jiang 2019 {published data only}
-
- Jiang W, Wang L, Yongtao L, Zhang C, Yi L, Ou J. Clinical application of docetaxel combined with lobaplatin in adjuvant chemotherapy of elderly triple-negative breast cancer. Anti-tumor Pharmacy 2019;9(6):897-901.
NCT00004092 {published data only}
-
- NCT00004092. Combination chemotherapy in treating patients with high-risk breast cancer. clinicaltrials.gov/ct2/show/NCT00004092 (first received 27 January 2003).
SICOG 9908 {published data only}
-
- Frasci G, D'Aiuto G, Comella P, D'Aiuto M, Di Bonito M, Ruffolo P, et al. Preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) improves prognosis in locally advanced breast cancer patients: an update of the Southern Italy Cooperative Oncology Group (SICOG) randomised trial 9908. Annals of Oncology 2010;21:707-16. - PubMed
-
- Frasci G, D'Aiuto G, Comella P, Thomas R, Botti G, Di Bonito M, et al. Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: final analysis of a SICOG phase II study. British Journal of Cancer 2006;95:1005-12. - PMC - PubMed
UMIN000030780 – jRCTs051180210 {published data only}
-
- UMIN000030780. Phase 3 trial of carboplatin in triple negative breast cancer patient with residual invasive carcinoma after neoadjuvant chemotherapy. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000034530 (first received 1 March 2018).
-
- jRCTs051180210. Phase 3 trial of carboplatin in triple negative breast cancer patient with residual invasive carcinoma after neoadjuvant chemotherapy. jrct.niph.go.jp/latest-detail/jRCTs051180210 (first received 27 March 2019).
References to ongoing studies
ChiCTR1800019501 {published data only}
-
- ChiCTR1800019501. A randomized controlled phase II clinical trial comparing neoadjuvant TP (docetaxel + cisplatin) with TAC (docetaxel + adriamycin + cyclophosphamide) regimen in the treatment of operable triple negative breast cancer. www.chictr.org.cn/showprojEN.html?proj=31567 (first received 14 November 2018).
ChiCTR1900023776 {published data only}
-
- ChiCTR1900023776. A randomized, controlled, single-center clinical study for the efficacy and safety of docetaxel plus lobaplatin versus docetaxel plus epirubicin for neoadjuvant therapy in triple-negative breast cancer. www.chictr.org.cn/showprojEN.html?proj=39908 (first received 11 June 2019).
ChiCTR1900026499 {published data only}
-
- ChiCTR1900026499. Albumin-bound paclitaxel combined with carboplatin versus epirubicin combined with docetaxel as neoadjuvant therapy for triple-negative breast cancer: a multicenter randomized controlled phase IV clinical trial. www.chictr.org.cn/showprojEN.html?proj=44204 (first received 12 October 2019).
ChiCTR2000039578 {published data only}
-
- ChiCTR2000039578. A prospective, open, multicenter, randomized controlled trial for the effect of albumin-bound paclitaxel combined with cisplatin versus epirubicin combined with cyclophosphamide sequential docetaxel neoadjuvant therapy for triple negative breast cancer. www.chictr.org.cn/showprojEN.html?proj=63610 (first received 1 November 2020).
CTRI/2017/10/010272 {published data only}
-
- CTRI/2017/10/010272. Comparing two types of chemotherapy in breast cancer. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=20743 (first received 31 October 2017).
CTRI/2019/05/019176 {published data only}
-
- CTRI/2019/05/019176. A trial comparing effect of addition of carboplatin to standard neoadjuvant chemotherapy for triple negative breast cancer patients. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=32946 (first received 16 May 2019).
EUCTR2009‐015238‐31 {published data only}
-
- EUCTR2009-015238-31. Randomized phase II/III study of individualized neoadjuvant chemotherapy in 'triple-negative' breast tumors. www.clinicaltrialsregister.eu/ctr-search/search?query=2009-015238-31 (first received 16 December 2009).
-
- NCT01057069. Neo adjuvant chemotherapy in triple negative breast cancer (neo-TN). clinicaltrials.gov/ct2/show/NCT01057069 (first received 27 January 2010).
EudraCT2016‐004384‐39 {published data only}
-
- EudraCT2016-004384-39. A clinical trial that examines whether the treatment with the medication olaparib in combination with the chemotherapy carboplatin is more effective than treatment with a standard chemotherapy (anthracycline/ taxane-based) against a specific type of breast cancer. www.clinicaltrialsregister.eu/ctr-search/trial/2016-004384-39/AT (first received 6 November 2018).
NCT00919880 {published data only}
-
- NCT00919880. Comparison of neo-adjuvant weekly paclitaxel with or without carboplatin in early breast cancer. clinicaltrials.gov/show/NCT00919880 (first received 12 June 2009).
NCT01752686 {published data only}
-
- NCT01752686. A phase III trial of carboplatin as adjuvant chemotherapy in triple negative breast cancer. clinicaltrials.gov/show/NCT01752686 (first received 19 December 2012).
NCT02041338 {published data only}
-
- NCT02041338. Study of optimizing neoadjuvant regimens in subtypes of breast cancer. clinicaltrials.gov/show/NCT02041338 (first received 22 January 2014).
NCT02455141 {published data only}
-
- NCT02455141. Adjuvant treatment of EC followed by taxane +/- carboplatin in triple-negative breast cancer. clinicaltrials.gov/show/NCT02455141 (first received 27 May 2015).
NCT02488967 {published data only}
-
- NCT02488967. Doxorubicin hydrochloride and cyclophosphamide followed by paclitaxel with or without carboplatin in treating patients with triple-negative breast cancer. clinicaltrials.gov/show/NCT02488967 (first received 2 July 2015).
NCT02641847 {published data only}
-
- NCT02641847. TA(E)C-GP versus A(E)C-T for the high risk TNBC patients and validation of the mRNA-lncRNA signature. clinicaltrials.gov/show/NCT02641847 (first received 30 December 2015).
NCT02879513 {published data only}
-
- NCT02879513. Trial of adjuvant chemotherapy in breast cancer patients with pathological partial response and complete response to neoadjuvant chemotherapy. clinicaltrials.gov/show/NCT02879513 (first received 25 August 2016).
NCT02978495 {published data only}
-
- NCT02978495. Neoadjuvant carboplatin in triple negative breast cancer. clinicaltrials.gov/show/NCT02978495 (first received 1 December 2016).
NCT03168880 {published data only}
-
- NCT03168880. A randomized controlled trial of neoadjuvant weekly paclitaxel versus weekly paclitaxel plus weekly carboplatin in women with large operable or locally advanced, triple negative breast cancer (TNBC). clinicaltrials.gov/ct2/show/NCT03168880 (first received 30 May 2017).
NCT03201861 {published data only}
-
- NCT03201861. Addition of cisplatin to adjuvant chemotherapy for early stage breast cancer in high-risk women. clinicaltrials.gov/show/NCT03201861 (first received 28 June 2017).
NCT03876886 {published data only}
-
- NCT03876886. The trial comparing dose-dense AC-T with TP as adjuvant therapy for TNBC with homologous recombination repair deficiency. clinicaltrials.gov/show/NCT03876886 (first received 15 March 2019).
NCT04136782 {published data only}
-
- NCT04136782. Albumin-bound paclitaxel and carboplatin versus epirubicin and docetaxel for triple-negative breast cancer. clinicaltrials.gov/show/NCT04136782 (first received 23 October 2019).
NCT04138719 {published data only}
-
- NCT04138719. Nab-paclitaxel plus carboplatin versus nab-paclitaxel plus epirubicin in the neoadjuvant therapy for breast cancer. clinicaltrials.gov/show/NCT04138719 (first received 24 October 2019).
NCT04296175 {published data only}
-
- NCT04296175. Carboplatin intensified chemotherapy for triple negative breast cancer (CITRINE). clinicaltrials.gov/show/NCT04296175 (first received 5 March 2020).
NCT04664972 {published data only}
-
- NCT04664972. The TP regimen in the treatment of early triple negative breast cancer. clinicaltrials.gov/show/NCT04664972 (first received 11 December 2020).
Nordic Trip {published data only}
-
- Loman N, Linderholm B, Joensuu H, Ejlertsen B, Johannsson OT, Geisler J, et al. Abstract OT3-02-10: Nordic trip, a randomized phase 3 study in early triple negative breast cancer. Cancer Research 2016;76(4 Suppl 1):OT3-02-10.
PEARLY {published data only}
-
- KCT0003529. A randomized, multicenter, open-label, phase III trial comparing anthracyclines followed by taxane versus anthracyclines followed by taxane plus carboplatin as (neo)adjuvant therapy in patients with triple-negative breast cancer. cris.nih.go.kr/cris/search/detailSearch.do?seq=14516&search_page=L&a... (first received 23 November 2018).
-
- Kim GM, Jeung HC, Jung KH, Kim SH, Kim HJ, Lee KH, et al. PEARLY: a randomized, multicenter, open-label, phase III trial comparing anthracyclines followed by taxane versus anthracyclines followed by taxane plus carboplatin as (neo)adjuvant therapy in patients with early triple-negative breast cancer. Journal of Clinical Oncology 2017;35(15 Suppl 1):TPS587.
-
- NCT02441933. Carboplatin in EARLY triple negative breast cancer trial (PEARLY trial). clinicaltrials.gov/show/NCT02441933 (first received 12 May 2015).
Additional references
Awidi 2021
Banys‐Paluchowski 2017
Bian 2021
BrighTNess
-
- Loibl S, O'Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncology 2018;19(4):497-509. - PubMed
Cortazar 2014
-
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384(9938):164-72. - PubMed
CTCAE 2017
-
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v... (accessed 25 March 2021).
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. - PubMed
Edge 2010
-
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology 2010;17(6):1471-4. - PubMed
Ferlay 2018
-
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al, editor(s). Cancer Today (powered by GLOBOCAN 2018) IARC CancerBase No. 15. publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Today-Powered-By-... (accessed 17 July 2020).
Foulkes 2010
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New England Journal of Medicine 2010;363(20):1938-48. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed prior to 4 September 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guo 2017
-
- Guo W, Lin L, He X, He F, Wang C, Chen N, et al. Biomarkers of DNA repair and related pathways: significance of treatment in triple-negative breast cancer. Critical Reviews in Oncogenesis 2017;22(5-6):427-37. - PubMed
Gupta 2022
-
- Gupta S, Nair NS, Hawaldar RW, Vanmali V, Parmar V, Gulia S, et al. Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: a phase III randomized controlled trial. San Antonio Breast Cancer Symposium; 2022 Dec 6-10; San Antonio, Texas.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
-
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Lin 2012
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719-48. - PubMed
McGlothlin 2014
-
- McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA 2014;312(13):1342-3. - PubMed
Nitz 2019
-
- Nitz U, Gluz O, Clemens M, Malter W, Reimer T, Nuding B, et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. Journal of Clinical Oncology 2019;37(10):799-808. - PubMed
O'Reilly 2020
-
- O'Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. Journal of Clinical Oncology 2020;38(13):1378-88. - PMC - PubMed
Pandy 2019
Pennington 2014
Perabo 2007
-
- Perabo FG, Müller SC. New agents for treatment of advanced transitional cell carcinoma. Annals of Oncology 2007;18:835-43. - PubMed
Petrelli 2014
-
- Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Research and Treatment 2014;144(2):223-32. - PubMed
Poggio 2018
-
- Poggio F, Bruzzone M, Ceppi M, Ponde NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Annals of Oncology 2018;29(7):1497-508. - PubMed
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 2.4.2. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Saleh 2021
-
- Saleh RR, Nadler MB, Desnoyers A, Meti N, Fazelzad R, Amir E. Platinum-based chemotherapy in early-stage triple negative breast cancer: a meta-analysis. Cancer Treatment Reviews 2021;100:102283. - PubMed
Schmid 2020
-
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. New England Journal of Medicine 2020;382(9):810-21. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shepherd 2022
-
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. - PMC - PubMed
Shimelis 2018
Tierney 2007
Tutt 2021
Wang 2017
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

