Considerations for defining +80 Da mass shifts in mass spectrometry-based proteomics: phosphorylation and beyond
- PMID: 37681662
- PMCID: PMC10521633
- DOI: 10.1039/d3cc02909c
Considerations for defining +80 Da mass shifts in mass spectrometry-based proteomics: phosphorylation and beyond
Abstract
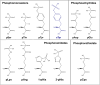
Post-translational modifications (PTMs) are ubiquitous and key to regulating protein function. Understanding the dynamics of individual PTMs and their biological roles requires robust characterisation. Mass spectrometry (MS) is the method of choice for the identification and quantification of protein modifications. This article focusses on the MS-based analysis of those covalent modifications that induce a mass shift of +80 Da, notably phosphorylation and sulfation, given the challenges associated with their discrimination and pinpointing the sites of modification on a polypeptide chain. Phosphorylation in particular is highly abundant, dynamic and can occur on numerous residues to invoke specific functions, hence robust characterisation is crucial to understanding biological relevance. Showcasing our work in the context of other developments in the field, we highlight approaches for enrichment and site localisation of phosphorylated (canonical and non-canonical) and sulfated peptides, as well as modification analysis in the context of intact proteins (top down proteomics) to explore combinatorial roles. Finally, we discuss the application of native ion-mobility MS to explore the effect of these PTMs on protein structure and ligand binding.
Conflict of interest statement
There are no conflicts to declare.
Figures












References
-
- Aebersold R. Agar J. N. Amster I. J. Baker M. S. Bertozzi C. R. Boja E. S. Costello C. E. Cravatt B. F. Fenselau C. Garcia B. A. Ge Y. Gunawardena J. Hendrickson R. C. Hergenrother P. J. Huber C. G. Ivanov A. R. Jensen O. N. Jewett M. C. Kelleher N. L. Kiessling L. L. Krogan N. J. Larsen M. R. Loo J. A. Ogorzalek Loo R. R. Lundberg E. MacCoss M. J. Mallick P. Mootha V. K. Mrksich M. Muir T. W. Patrie S. M. Pesavento J. J. Pitteri S. J. Rodriguez H. Saghatelian A. Sandoval W. Schlüter H. Sechi S. Slavoff S. A. Smith L. M. Snyder M. P. Thomas P. M. Uhlén M. Van Eyk J. E. Vidal M. Walt D. R. White F. M. Williams E. R. Wohlschlager T. Wysocki V. H. Yates N. A. Young N. L. Zhang B. Nat. Chem. Biol. 2018;14:206–214. doi: 10.1038/nchembio.2576. - DOI - PMC - PubMed
-
- Eyers C. E. Gaskell S. J. Wiley Encycl. Chem. Biol. 2007:1–34. doi: 10.1002/9780470048672.wecb469. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

