Integrins and Actions of Androgen in Breast Cancer
- PMID: 37681860
- PMCID: PMC10486718
- DOI: 10.3390/cells12172126
Integrins and Actions of Androgen in Breast Cancer
Abstract
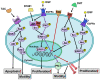
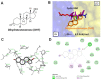
Androgen has been shown to regulate male physiological activities and cancer proliferation. It is used to antagonize estrogen-induced proliferative effects in breast cancer cells. However, evidence indicates that androgen can stimulate cancer cell growth in estrogen receptor (ER)-positive and ER-negative breast cancer cells via different types of receptors and different mechanisms. Androgen-induced cancer growth and metastasis link with different types of integrins. Integrin αvβ3 is predominantly expressed and activated in cancer cells and rapidly dividing endothelial cells. Programmed death-ligand 1 (PD-L1) also plays a vital role in cancer growth. The part of integrins in action with androgen in cancer cells is not fully mechanically understood. To clarify the interactions between androgen and integrin αvβ3, we carried out molecular modeling to explain the potential interactions of androgen with integrin αvβ3. The androgen-regulated mechanisms on PD-L1 and its effects were also addressed.
Keywords: PD-L; androgen; androgen receptor; breast cancer; integrin αvβ3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Feng Y., Spezia M., Huang S., Yuan C., Zeng Z., Zhang L., Ji X., Liu W., Huang B., Luo W., et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

