Recruitment of the Histone Variant MacroH2A1 to the Pericentric Region Occurs upon Chromatin Relaxation and Is Responsible for Major Satellite Transcriptional Regulation
- PMID: 37681907
- PMCID: PMC10486525
- DOI: 10.3390/cells12172175
Recruitment of the Histone Variant MacroH2A1 to the Pericentric Region Occurs upon Chromatin Relaxation and Is Responsible for Major Satellite Transcriptional Regulation
Abstract
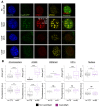
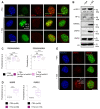
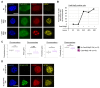
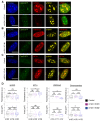
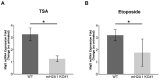
Heterochromatin formation plays a pivotal role in regulating chromatin organization and influences nuclear architecture and genome stability and expression. Amongst the locations where heterochromatin is found, the pericentric regions have the capability to attract the histone variant macroH2A1. However, the factors and mechanisms behind macroH2A1 incorporation into these regions have not been explored. In this study, we probe different conditions that lead to the recruitment of macroH2A1 to pericentromeric regions and elucidate its underlying functions. Through experiments conducted on murine fibroblastic cells, we determine that partial chromatin relaxation resulting from DNA damage, senescence, or histone hyper-acetylation is necessary for the recruitment of macroH2A1 to pericentric regions. Furthermore, macroH2A1 is required for upregulation of noncoding pericentric RNA expression but not for pericentric chromatin organization. Our findings shed light on the functional rather than structural significance of macroH2A1 incorporation into pericentric chromatin.
Keywords: chromatin decondensation; histone variant; macroH2A1; pericentromeric regions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

