Safety and efficacy of zanubrutinib in relapsed/refractory marginal zone lymphoma: final analysis of the MAGNOLIA study
- PMID: 37682792
- PMCID: PMC10679804
- DOI: 10.1182/bloodadvances.2023010668
Safety and efficacy of zanubrutinib in relapsed/refractory marginal zone lymphoma: final analysis of the MAGNOLIA study
Abstract
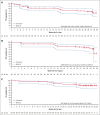
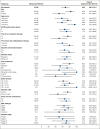
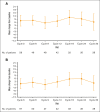
The primary analysis of MAGNOLIA, an open-label, single-arm, multicenter, phase 2 study, demonstrated that the next-generation Bruton tyrosine kinase (BTK) inhibitor zanubrutinib provided a high overall response rate (ORR) in patients with relapsed/refractory marginal zone lymphoma (R/R MZL), with a favorable safety/tolerability profile. Presented here, is the final analysis of MAGNOLIA, performed to characterize the durability of response and longer-term safety and tolerability. Zanubrutinib (160 mg twice daily) was evaluated in 68 patients with R/R MZL who had received at least 1 anti-CD20-directed regimen. The primary end point was independent review committee (IRC)-assessed ORR. Secondary end points included investigator-assessed ORR, duration of response (DOR), progression-free survival (PFS), overall survival (OS), health-related quality of life, safety, and tolerability. With a median follow-up of 27.4 months, the IRC-assessed ORR was 68.2% (95% confidence interval [CI], 55.6-79.1), with a 24-month DOR event-free rate of 72.9% (95% CI, 54.4-84.9). PFS and OS at 24 months were 70.9% (95% CI, 57.2-81.0) and 85.9% (95% CI, 74.7-92.4), respectively. The zanubrutinib safety profile was consistent with the primary analysis, with no new safety signals observed. Atrial fibrillation/flutter (n = 2 [2.9%]) and hypertension (n = 3 [4.4%]) were uncommon. Neutropenia (n = 8 [11.8%]) was the most common grade ≥3 adverse event. In this final analysis of MAGNOLIA, zanubrutinib demonstrated sustained clinical responses beyond 2 years, with 73% of responders alive and progression free. Zanubrutinib continued to demonstrate a favorable safety/tolerability profile with the additional time on treatment. This trial was registered at www.clinicaltrials.gov as #NCT03846427.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.O. has acted as a consultant/adviser for AbbVie, BeiGene, Janssen, Gilead, Roche, Mundipharma, Merck, and Bristol Myers Squibb; has received research funding from AbbVie, BeiGene, Janssen, Gilead, Roche, and Epizyme; and has received honoraria from AbbVie, BeiGene, Janssen, Gilead, Roche, Merck, and Bristol Myers Squibb. A.T. reports consulting fees from AbbVie, AstraZeneca, and BeiGene; payment of honoraria for lectures, presentations, speaker’s bureaus, and manuscript writing of educational events from AbbVie, AstraZeneca, and BeiGene; and support for attending meetings and/or travel from Janssen, AstraZeneca, and AbbVie. B.H. reports receiving consulting fees from BeiGene, ADC Therapeutics, Janssen, and Incyte. K.M.L. reports payment for expert testimony at the National Institute for Health and Care Excellence Office for Market Access (Zanubrutinib in MZL) and as a member of BeiGene consultancy advisory boards. P.M. reports consulting fees from AbbVie, AstraZeneca, BeiGene, Celgene/ Bristol Myers Squibb, Epizyme, Gilead/Kite, Incyte, Janssen, Roche, and Takeda; payment of honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Gilead/Kite, Incyte, and Janssen; support for attending meetings and/or travel from Takeda and Janssen; and participation in noncommercial trials. P.L.Z. reports consulting fees from Takeda, Roche, Janssen, Merck Sharpe & Dohme, Novartis, Gilead, Kyowa Kirin, Incyte, and BeiGene; and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Takeda, Sanofi, BeiGene, AstraZeneca, Gilead, Novartis, Bristol Myers Squibb, Roche, Kyowa Kirin, Incyte, Janssen, and Merck Sharpe & Dohme. C.T. reports payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Kite, Novartis, Roche, Incyte, AbbVie, Gilead, Roche, and Janssen; and support for attending meetings and/or travel from Kite, Novartis, Roche, Incyte, AbbVie, Gilead, Roche, and Janssen. K.A. reports support for attending meetings and/or travel from Gilead, Novartis, and Bristol Myers Squibb. P.W. reports consulting or advisory roles for BeiGene and Acerta. E.A.H. reports research funding to her institution from Roche, Bristol Myers Squibb, Merck KGaA, AstraZeneca, and Merck; and advisory board and speaker fees (institutional or personal) from Roche, Merck Sharpe & Dohme, AstraZeneca, Gilead, Antigene, Novartis, Regeneron, Janssen, and Specialised Therapeutics, outside the submitted work. Z.L., J.X., C.T., R.D., and M. Co are employees of, and own stock in, BeiGene Co, Ltd. J.T. reports research funding to her institution from BeiGene. The remaining authors declare no competing financial interests.
Figures

References
-
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. - PubMed
-
- National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: B-cell lymhomas, version 5. 2022. https://www.nccn.org/guidelines/category_1
-
- LaCasce AS, Noy A. Updates to the management of patients with relapsed or refractory indolent follicular and marginal zone lymphomas. J Natl Compr Canc Netw. 2022;20(5.5):578–580.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

