TNFα is a key trigger of inflammation in diet-induced non-obese MASLD in mice
- PMID: 37683301
- PMCID: PMC10493600
- DOI: 10.1016/j.redox.2023.102870
TNFα is a key trigger of inflammation in diet-induced non-obese MASLD in mice
Abstract
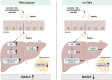
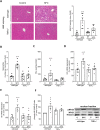
Tumor necrosis factor alpha (TNFα) is thought to be a critical factor in the development of metabolic dysfunction-associated steatotic liver disease (MASLD). Here, we determined the effects of a treatment with the anti-TNFα antibody infliximab and a genetic deletion of TNFα, respectively, in the development of non-obese diet-induced early metabolic dysfunction-associated steatohepatitis (MASH) in mice. The treatment with infliximab improved markers of liver damage in mice with pre-existing early MASH. In TNFα-/- mice, the development of early signs of MASH and insulin resistance was significantly attenuated compared to wild-type animals. While mRNA expression of proinflammatory cytokines like interleukin 1β (Il1b) and interleukin 6 (Il6) were significantly lower in livers of MASH-diet-fed TNFα-/- mice compared to wild-type mice with early MASH, markers of intestinal barrier function were similarly impaired in both MASH-diet-fed groups compared to controls. Our data suggest that TNFα is a key regulator of hepatic inflammation and insulin resistance associated with the development of early non-obese MASH.
Keywords: Endotoxin; Fatty liver; Insulin resistance; Intestinal barrier; MASH.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors declare no conflict of interest.
Figures



References
-
- Rinella M.E., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2023 - PubMed
-
- Riazi K., et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. - PubMed
-
- Chakravarthy M.V., et al. Nutrition and nonalcoholic fatty liver disease: current perspectives. Gastroenterol. Clin. N. Am. 2020;49:63–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

