Lgr5-expressing secretory cells form a Wnt inhibitory niche in cartilage critical for chondrocyte identity
- PMID: 37683603
- PMCID: PMC10790417
- DOI: 10.1016/j.stem.2023.08.004
Lgr5-expressing secretory cells form a Wnt inhibitory niche in cartilage critical for chondrocyte identity
Abstract
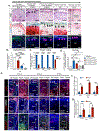
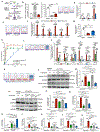
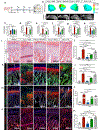
Osteoarthritis is a degenerative joint disease that causes pain, degradation, and dysfunction. Excessive canonical Wnt signaling in osteoarthritis contributes to chondrocyte phenotypic instability and loss of cartilage homeostasis; however, the regulatory niche is unknown. Using the temporomandibular joint as a model in multiple species, we identify Lgr5-expressing secretory cells as forming a Wnt inhibitory niche that instruct Wnt-inactive chondroprogenitors to form the nascent synovial joint and regulate chondrocyte lineage and identity. Lgr5 ablation or suppression during joint development, aging, or osteoarthritis results in depletion of Wnt-inactive chondroprogenitors and a surge of Wnt-activated, phenotypically unstable chondrocytes with osteoblast-like properties. We recapitulate the cartilage niche and create StemJEL, an injectable hydrogel therapy combining hyaluronic acid and sclerostin. Local delivery of StemJEL to post-traumatic osteoarthritic jaw and knee joints in rabbit, rat, and mini-pig models restores cartilage homeostasis, chondrocyte identity, and joint function. We provide proof of principal that StemJEL preserves the chondrocyte niche and alleviates osteoarthritis.
Keywords: Lgr5; WNT signaling; articular cartilage; biomaterials; chondroprogenitor cells; hydrogels; osteoarthritis; regeneration; skeletal development; stem cell niche; synovial joint; temporomandibular joint.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.C.E. and M.C. are inventors of a patent application on an osteoarthritis therapy related to this work (US patent 17/253993), which has been licensed to Wnt Scientific for commercialization. M.C. is a founder and equity holder of Wnt Scientific. H.Y. is an advisor and equity holder of Wnt Scientific.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

