Plasma glycoproteomics delivers high-specificity disease biomarkers by detecting site-specific glycosylation abnormalities
- PMID: 37683725
- PMCID: PMC11258658
- DOI: 10.1016/j.jare.2023.09.002
Plasma glycoproteomics delivers high-specificity disease biomarkers by detecting site-specific glycosylation abnormalities
Abstract
Introduction: The human plasma glycoproteome holds enormous potential to identify personalized biomarkers for diagnostics. Glycoproteomics has matured into a technology for plasma N-glycoproteome analysis but further evolution towards clinical applications depends on the clinical validity and understanding of protein- and site-specific glycosylation changes in disease.
Objectives: Here, we exploited the uniqueness of a patient cohort of genetic defects in well-defined glycosylation pathways to assess the clinical applicability of plasma N-glycoproteomics.
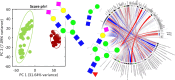
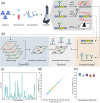
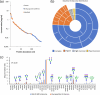
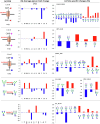
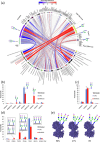
Methods: Comparative glycoproteomics was performed of blood plasma from 40 controls and 74 patients with 13 different genetic diseases that impact the protein N-glycosylation pathway. Baseline glycosylation in healthy individuals was compared to reference glycome and intact transferrin protein mass spectrometry data. Use of glycoproteomics data for biomarker discovery and sample stratification was evaluated by multivariate chemometrics and supervised machine learning. Clinical relevance of site-specific glycosylation changes were evaluated in the context of genetic defects that lead to distinct accumulation or loss of specific glycans. Integrated analysis of site-specific glycoproteome changes in disease was performed using chord diagrams and correlated with intact transferrin protein mass spectrometry data.
Results: Glycoproteomics identified 191 unique glycoforms from 58 unique peptide sequences of 34 plasma glycoproteins that span over 3 magnitudes of abundance in plasma. Chemometrics identified high-specificity biomarker signatures for each of the individual genetic defects with better stratification performance than the current diagnostic standard method. Bioinformatic analyses revealed site-specific glycosylation differences that could be explained by underlying glycobiology and protein-intrinsic factors.
Conclusion: Our work illustrates the strong potential of plasma glycoproteomics to significantly increase specificity of glycoprotein biomarkers with direct insights in site-specific glycosylation changes to better understand the glycobiological mechanisms underlying human disease.
Keywords: Blood plasma; Clinical applications; Congenital disorders of glycosylation; Glycoproteomics; Glycosylation.
Copyright © 2024. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Contributing authors Pierre-Olivier Schmit, Stuart Pengelley, and Kristina Marx are employees of Bruker Daltonics which is the manufacturer of some of the hardware and software that were used in this work.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

