Training-induced circuit-specific excitatory synaptogenesis in mice is required for effort control
- PMID: 37684234
- PMCID: PMC10491649
- DOI: 10.1038/s41467-023-41078-z
Training-induced circuit-specific excitatory synaptogenesis in mice is required for effort control
Abstract
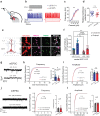
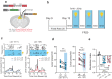
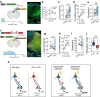
Synaptogenesis is essential for circuit development; however, it is unknown whether it is critical for the establishment and performance of goal-directed voluntary behaviors. Here, we show that operant conditioning via lever-press for food reward training in mice induces excitatory synapse formation onto a subset of anterior cingulate cortex neurons projecting to the dorsomedial striatum (ACC→DMS). Training-induced synaptogenesis is controlled by the Gabapentin/Thrombospondin receptor α2δ-1, which is an essential neuronal protein for proper intracortical excitatory synaptogenesis. Using germline and conditional knockout mice, we found that deletion of α2δ-1 in the adult ACC→DMS circuit diminishes training-induced excitatory synaptogenesis. Surprisingly, this manipulation does not impact learning but results in a significant increase in effort exertion without affecting sensitivity to reward value or changing contingencies. Bidirectional optogenetic manipulation of ACC→DMS neurons rescues or phenocopies the behaviors of the α2δ-1 cKO mice, highlighting the importance of synaptogenesis within this cortico-striatal circuit in regulating effort exertion.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

