Continuous peripersonal tracking accuracy is limited by the speed and phase of locomotion
- PMID: 37684285
- PMCID: PMC10491677
- DOI: 10.1038/s41598-023-40655-y
Continuous peripersonal tracking accuracy is limited by the speed and phase of locomotion
Abstract
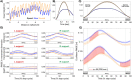
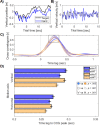
Recent evidence suggests that perceptual and cognitive functions are codetermined by rhythmic bodily states. Prior investigations have focused on the cardiac and respiratory rhythms, both of which are also known to synchronise with locomotion-arguably our most common and natural of voluntary behaviours. Compared to the cardiorespiratory rhythms, walking is easier to voluntarily control, enabling a test of how natural and voluntary rhythmic action may affect sensory function. Here we show that the speed and phase of human locomotion constrains sensorimotor performance. We used a continuous visuo-motor tracking task in a wireless, body-tracking virtual environment, and found that the accuracy and reaction time of continuous reaching movements were decreased at slower walking speeds, and rhythmically modulated according to the phases of the step-cycle. Decreased accuracy when walking at slow speeds suggests an advantage for interlimb coordination at normal walking speeds, in contrast to previous research on dual-task walking and reach-to-grasp movements. Phasic modulations of reach precision within the step-cycle also suggest that the upper limbs are affected by the ballistic demands of motor-preparation during natural locomotion. Together these results show that the natural phases of human locomotion impose constraints on sensorimotor function and demonstrate the value of examining dynamic and natural behaviour in contrast to the traditional and static methods of psychological science.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Engel AK, Maye A, Kurthen M, König P. Where’s the action? The pragmatic turn in cognitive science. Trends Cogn. Sci. 2013;17(5):202–209. - PubMed
-
- Herwig A. Linking perception and action by structure or process? Toward an integrative perspective. Neurosci. Biobehav. Rev. 2015;52:105–116. - PubMed
-
- Hommel B, Wiers RW. Towards a unitary approach to human action control. Trends Cogn. Sci. 2017;21(12):940–949. - PubMed
-
- O’Regan JK. Solving the "real" mysteries of visual perception: The world as an outside memory. Can. J. Psychol. 1992;46(3):461–488. - PubMed
-
- O’Regan JK, Noë A. A sensorimotor account of vision and visual consciousness. Behav. Brain Sci. 2001;24(5):939–973. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

