A disease-specific iPS cell resource for studying rare and intractable diseases
- PMID: 37684663
- PMCID: PMC10485998
- DOI: 10.1186/s41232-023-00294-2
A disease-specific iPS cell resource for studying rare and intractable diseases
Abstract
Background: Disease-specific induced pluripotent stem cells (iPSCs) are useful tools for pathological analysis and diagnosis of rare diseases. Given the limited available resources, banking such disease-derived iPSCs and promoting their widespread use would be a promising approach for untangling the mysteries of rare diseases. Herein, we comprehensively established iPSCs from patients with designated intractable diseases in Japan and evaluated their properties to enrich rare disease iPSC resources.
Methods: Patients with designated intractable diseases were recruited for the study and blood samples were collected after written informed consent was obtained from the patients or their guardians. From the obtained samples, iPSCs were established using the episomal method. The established iPSCs were deposited in a cell bank.
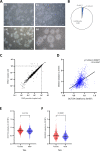
Results: We established 1,532 iPSC clones from 259 patients with 139 designated intractable diseases. The efficiency of iPSC establishment did not vary based on age and sex. Most iPSC clones originated from non-T and non-B hematopoietic cells. All iPSC clones expressed key transcription factors, OCT3/4 (range 0.27-1.51; mean 0.79) and NANOG (range 0.15-3.03; mean 1.00), relative to the reference 201B7 iPSC clone.
Conclusions: These newly established iPSCs are readily available to the researchers and can prove to be a useful resource for research on rare intractable diseases.
Keywords: Designated diseases; Rare and intractable diseases; Reprogramming; iPS cells.
© 2023. Japanese Society of Inflammation and Regeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Grants and funding
- JP21bm0104001/Japan Agency for Medical Research and Development
- JP21bm0104001/Japan Agency for Medical Research and Development
- 16bm0609001h0005/Japan Agency for Medical Research and Development
- 16bm0609001h0005/Japan Agency for Medical Research and Development
- JP21bm0804004/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Research Materials

