Fluid shear stress induces a shift from glycolytic to amino acid pathway in human trophoblasts
- PMID: 37684702
- PMCID: PMC10492287
- DOI: 10.1186/s13578-023-01114-3
Fluid shear stress induces a shift from glycolytic to amino acid pathway in human trophoblasts
Abstract
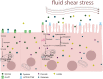
Background: The human placenta, a tissue with a lifespan limited to the period of pregnancy, is exposed to varying shear rates by maternal blood perfusion depending on the stage of development. In this study, we aimed to investigate the effects of fluidic shear stress on the human trophoblast transcriptome and metabolism.
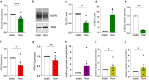
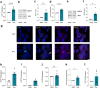
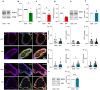
Results: Based on a trophoblast cell line cultured in a fluidic flow system, changes caused by shear stress were analyzed and compared to static conditions. RNA sequencing and bioinformatics analysis revealed an altered transcriptome and enriched gene ontology terms associated with amino acid and mitochondrial metabolism. A decreased GLUT1 expression and reduced glucose uptake, together with downregulated expression of key glycolytic rate-limiting enzymes, hexokinase 2 and phosphofructokinase 1 was observed. Altered mitochondrial ATP levels and mass spectrometry data, suggested a shift in energy production from glycolysis towards mitochondrial oxidative phosphorylation. This shift in energy production could be supported by increased expression of glutamic-oxaloacetic transaminase variants in response to shear stress as well as under low glucose availability or after silencing of GLUT1. The shift towards amino acid metabolic pathways could be supported by significantly altered amino acid levels, like glutamic acid, cysteine and serine. Downregulation of GLUT1 and glycolytic rate-limiting enzymes, with concomitant upregulation of glutamic-oxaloacetic transaminase 2 was confirmed in first trimester placental explants cultured under fluidic flow. In contrast, high fluid shear stress decreased glutamic-oxaloacetic transaminase 2 expression in term placental explants when compared to low flow rates. Placental tissue from pregnancies with intrauterine growth restriction are exposed to high shear rates and showed also decreased glutamic-oxaloacetic transaminase 2, while GLUT1 was unchanged and glycolytic rate-limiting enzymes showed a trend to be upregulated. The results were generated by using qPCR, immunoblots, quantification of immunofluorescent pictures, padlock probe hybridization, mass spectrometry and FRET-based measurement.
Conclusion: Our study suggests that onset of uteroplacental blood flow is accompanied by a shift from a predominant glycolytic- to an alternative amino acid converting metabolism in the villous trophoblast. Rheological changes with excessive fluidic shear stress at the placental surface, may disrupt this alternative amino acid pathway in the syncytiotrophoblast and could contribute to intrauterine growth restriction.
Keywords: Fluidic shear stress; Placenta development; Trophoblast metabolism.
© 2023. Society of Chinese Bioscientists in America (SCBA).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Fowden AL, Camm EJ, Sferruzzi-Perri AN. Effects of maternal obesity on placental phenotype. Curr Vasc Pharmacol. 2021;19:113–131. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

