Reduced Nitric Oxide Synthase Involvement in Aigamo Duck Basilar Arterial Relaxation
- PMID: 37685004
- PMCID: PMC10486467
- DOI: 10.3390/ani13172740
Reduced Nitric Oxide Synthase Involvement in Aigamo Duck Basilar Arterial Relaxation
Abstract
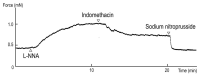
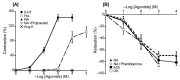
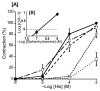
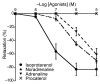
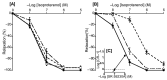
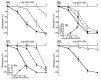
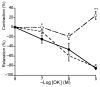
The basilar arterial endothelium mediates blood vessel relaxation partly through the release of nitric oxide (NO). Apoptosis of cerebrovascular endothelial cells is linked to a high mortality rate in chickens infected with the highly pathogenic avian influenza virus, but interestingly, ducks exhibit a greater resistance to this virus. In this study, we examined the responsiveness of duck basilar arteries (BAs) to various vasoactive substances, including 5-hydroxytryptamine (5-HT), histamine (His), angiotensin (Ang) II, noradrenaline (NA), acetylcholine (ACh), and avian bradykinin ornithokinin (OK), aiming to characterize the receptor subtypes involved and the role of endothelial NO in vitro. Our findings suggest that arterial contraction is mediated with 5-HT1 and H1 receptors, while relaxation is induced with β3-adrenergic and M3 receptors. Additionally, OK elicited a biphasic response in duck BAs, and Ang II had no effect. Endothelial NO appears to be crucial in relaxation mediated with M3 and OK receptors but not β3-adrenergic receptors in the duck BA. The reduced endothelial NO involvement in the receptor-mediated relaxation response in duck BAs represents a clear difference from the corresponding response reported in chicken BAs. This physiological difference may explain the differences in lethality between ducks and chickens when vascular endothelial cells are infected with the virus.
Keywords: cerebral artery; duck; endothelium; highly pathogenic avian influenza; nitric oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Vasomotor effects of noradrenaline, 5-hydroxytryptamine, angiotensin II, bradykinin, histamine, and acetylcholine on the bat (Rhinolophus ferrumequinum) basilar artery.Comp Biochem Physiol C Toxicol Pharmacol. 2021 Dec;250:109190. doi: 10.1016/j.cbpc.2021.109190. Epub 2021 Sep 15. Comp Biochem Physiol C Toxicol Pharmacol. 2021. PMID: 34536573
-
Vasomotor effects of 5-hydroxytryptamine, histamine, angiotensin II, acetylcholine, noradrenaline, and bradykinin on the cerebral artery of bottlenose dolphin (Tursiops truncatus).J Vet Med Sci. 2020 Oct 20;82(10):1456-1463. doi: 10.1292/jvms.20-0351. Epub 2020 Aug 19. J Vet Med Sci. 2020. PMID: 32814751 Free PMC article.
-
Vasomotor effects of noradrenaline, acetylcholine, histamine, 5-hydroxytryptamine and bradykinin on snake (Trimeresurus flavoviridis) basilar arteries.Comp Biochem Physiol C Toxicol Pharmacol. 2007 Nov;146(4):478-83. doi: 10.1016/j.cbpc.2007.05.007. Epub 2007 Jun 9. Comp Biochem Physiol C Toxicol Pharmacol. 2007. PMID: 17604230
-
Vasomotor effects of acetylcholine, bradykinin, noradrenaline, 5-hydroxytryptamine, histamine and angiotensin II on the mouse basilar artery.J Vet Med Sci. 2014 Oct;76(10):1339-45. doi: 10.1292/jvms.14-0223. Epub 2014 Jun 19. J Vet Med Sci. 2014. PMID: 24942113 Free PMC article.
-
Hypertension alters the endothelial-dependent biphasic response of bradykinin in isolated Microminipig basilar artery.Microvasc Res. 2017 Nov;114:52-57. doi: 10.1016/j.mvr.2017.06.001. Epub 2017 Jun 3. Microvasc Res. 2017. PMID: 28587989
References
-
- de Bruin A.C., Spronken M.I., Bestebroer T.M., Fouchier R.A., Richard M. Reduced replication of highly pathogenic avian influenza virus in duck endothelial cells compared to chicken endothelial cells is associated with stronger antiviral responses. Viruses. 2022;14:165. doi: 10.3390/v14010165. - DOI - PMC - PubMed
-
- Keawcharoen J., van Riel D., van Amerongen G., Bestebroer T., Beyer W.E., van Lavieren R., Osterhaus A.D., Fouchier R.A., Kuiken T. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1) Emerg. Infect. Dis. 2008;14:600–607. doi: 10.3201/eid1404.071016. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

