Conservative versus Invasive Strategy in Elderly Patients with Non-ST-Elevation Myocardial Infarction: Insights from the International POPular Age Registry
- PMID: 37685517
- PMCID: PMC10487667
- DOI: 10.3390/jcm12175450
Conservative versus Invasive Strategy in Elderly Patients with Non-ST-Elevation Myocardial Infarction: Insights from the International POPular Age Registry
Abstract
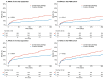
This registry assessed the impact of conservative and invasive strategies on major adverse clinical events (MACE) in elderly patients with non-ST-elevation myocardial infarction (NSTEMI). Patients aged ≥75 years with NSTEMI were prospectively registered from European centers and followed up for one year. Outcomes were compared between conservative and invasive groups in the overall population and a propensity score-matched (PSM) cohort. MACE included cardiovascular death, acute coronary syndrome, and stroke. The study included 1190 patients (median age 80 years, 43% female). CAG was performed in 67% (N = 798), with two-thirds undergoing revascularization. Conservatively treated patients had higher baseline risk. After propensity score matching, 319 patient pairs were successfully matched. MACE occurred more frequently in the conservative group (total population 20% vs. 12%, adjHR 0.53, 95% CI 0.37-0.77, p = 0.001), remaining significant in the PSM cohort (18% vs. 12%, adjHR 0.50, 95% CI 0.31-0.81, p = 0.004). In conclusion, an early invasive strategy was associated with benefits over conservative management in elderly patients with NSTEMI. Risk factors associated with ischemia and bleeding should guide strategy selection rather than solely relying on age.
Keywords: conservative strategy; coronary artery disease; elderly; invasive strategy; non-ST-elevation myocardial infarction.
Conflict of interest statement
JtB report grants from the Netherlands Organization for Health Research and Development, a Dutch government institution called ZonMw, and AstraZeneca; and personal fees from AstraZeneca, Boehringer Ingelheim, Bayer, Ferrer, Pfizer, and Merck, outside the submitted work. RFS reports research grants and personal fees from AstraZeneca, Cytosorbents, GlyCardial Diagnostics and Thromboserin; and personal fees from Alnylam, Bayer, Bristol Myers Squibb/Pfizer, Chiesi, CSL Behring, Daiichi Sankyo, HengRui, Idorsia, Intas Pharmaceuticals, Medscape, Novartis, PhaseBio, and Sanofi Aventis. DA reports research grants from TA Sciences, and personal fees from Philips/Volcano, Pfizer, Astra Zeneca and Novartis. AvH reports unrestricted grants to institution from Medtronic, AZ, Boehringer Ingelheim, Abbott. All other authors have no conflict of interests to declare.
Figures


References
-
- Eurostat Statistics Explained. Causes of Death Statistics. [(accessed on 11 January 2022)]. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cause....
-
- Husted S., James S., Becker R.C., Horrow J., Katus H., Storey R.F., Cannon C.P., Heras M., Lopes R.D., Morais J., et al. Ticagrelor versus Clopidogrel in Elderly Patients with Acute Coronary Syndromes: A Substudy from the Prospective Randomized PLATelet Inhibition and Patient Outcomes (PLATO) Trial. Circ. Cardiovasc. Qual. Outcomes. 2012;5:680–688. doi: 10.1161/CIRCOUTCOMES.111.964395. - DOI - PubMed
-
- Collet J.-P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2020;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. - DOI - PubMed
-
- Amsterdam E.A., Wenger N.K., Brindis R.G., Casey D.E., Ganiats T.G., Holmes D.R., Jaffe A.S., Jneid H., Kelly R.F., Kontos M.C., et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

