Clinical Utility of Pre-Therapeutic [18F]FDG PET/CT Imaging for Predicting Outcomes in Breast Cancer
- PMID: 37685551
- PMCID: PMC10488013
- DOI: 10.3390/jcm12175487
Clinical Utility of Pre-Therapeutic [18F]FDG PET/CT Imaging for Predicting Outcomes in Breast Cancer
Abstract
Background: [18F]FDG PET/CT is used for staging and could also provide information associated with clinical outcomes. The objective of this study was to determine the clinical utility of biomarkers measured using [18F]FDG PET/CT to predict the absence of pathological complete response (no-pCR) and recurrence.
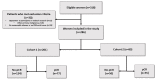
Methods: In this retrospective study, we included patients with non-special-type breast carcinoma who underwent [18F]FDG PET/CT before neoadjuvant chemotherapy between 2011 and 2019. Clinicopathological data were collected. Tumor SUVmax and total metabolic tumor volume (TMTV) were measured from PET images. The association between biomarkers and no-pCR was studied using logistic regression. The cut-off value was determined using the area under the ROC Curve. To predict 3-year recurrence-free survival (RFS), we used a multivariable Cox model, and the cut-off value was determined using time-dependent ROC and predictiveness curves.
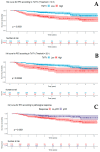
Results: Two hundred and eighty-six patients were included in the analysis. One hundred and twelve patients had a pCR (39.2%). The pCR rate was significantly higher in patients with a high nuclear grade (p < 0.01), HER2+ and TNBC subtypes (p < 0.01), high Ki67 (p < 0.01), and low TMTV (p < 0.01). A high TMTV value (>9.0 cm3) was significantly associated with no-pCR in the whole cohort (OR = 2.4, 95% CI: 1.3-4.2, p < 0.01). After a median follow-up of 4.5 years, 65 patients experienced recurrence and 39 patients died. High TMTV (>13.5 cm3) was associated with shorter RFS (HR = 4.0, 95% CI: 1.9-8.4, p < 0.01).
Conclusion: High TMTV in pre-therapeutic imaging is associated with no-pCR and recurrence. It can help in identifying high-risk patients and be considered as an intensified or alternative adjuvant therapy for closely monitoring patients.
Keywords: PET/CT imaging; breast cancer; neoadjuvant chemotherapy; pathological complete response; total metabolic tumor volume.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. doi: 10.1016/S1470-2045(17)30777-5. - DOI - PMC - PubMed
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

