Comparison of Clinical Outcomes after Platelet-Rich Plasma and Rotator Cuff Repair in High-Grade Intrasubstance Partial Rotator Cuff Tears
- PMID: 37685621
- PMCID: PMC10488403
- DOI: 10.3390/jcm12175554
Comparison of Clinical Outcomes after Platelet-Rich Plasma and Rotator Cuff Repair in High-Grade Intrasubstance Partial Rotator Cuff Tears
Abstract
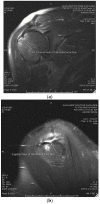
Platelet-rich plasma injections have been shown to have many useful applications in various musculoskeletal pathologies. Research on the use of PRP for intrasubstance partial-thickness rotator cuff tears is lacking, although these tears have unique properties that may increase the efficacy of platelet-rich plasma injections. Patients with MRI-confirmed high-grade intrasubstance partial-thickness rotator cuff tears, that had failed traditional non-operative treatment, were offered either surgical repair (Group 1) or a single ultrasound-guided platelet-rich plasma injection into the tear site (Group 2). Patients were followed at 2 weeks, 6 weeks, 3 months, and a minimum of 2 years post-injection with ASES scores. A total of 25 patients received platelet-rich plasma injections, compared to 20 patients who had rotator cuff repair for intrasubstance tears in the last 3 years. The mean pre-injection ASES score for the platelet-rich plasma group was 53.2 and this improved to 92.9 at a minimum 2-year follow-up. The average convalescence period following platelet-rich plasma injection was 3.3 months. The average post-operative convalescence period for arthroscopic rotator cuff repair was 4.6 months. Both surgical repair and platelet-rich plasma injection into the tear site are equally effective in the treatment of high-grade intrasubstance partial-thickness rotator cuff tears, while platelet-rich plasma provides significantly shorter recovery time.
Keywords: PRP; clinical outcomes; intrasubstance rotator cuff tears; partial rotator cuff tears; platelet-rich plasma; rotator cuff repair; rotator cuff tears.
Conflict of interest statement
Edwin Spencer received funding from Stryker Corporation (Kalamazoo, MI 49002, USA) and Encore Medical, LP (acquired by DJO LLC Carlsbad, CA 92010, USA). The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Benson Scott received funding from Medtronic, Inc. (Minneapolis, MN 55432, USA) and Siemens Medical Solutions USA, Inc. (Malvern, PA 19335, USA). The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Robert Sleadd PA-C received funding from Stryker Corporation (Kalamazoo, MI 49002, USA). The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. Grayson Poff declares no conflict of interest. John Broyles declares no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials