Influence of a Structured Microbiological Endotracheal Monitoring Program on the Outcome of Critically Ill COVID-19 Patients: An Observational Study
- PMID: 37685689
- PMCID: PMC10488947
- DOI: 10.3390/jcm12175622
Influence of a Structured Microbiological Endotracheal Monitoring Program on the Outcome of Critically Ill COVID-19 Patients: An Observational Study
Abstract
Background: In past influenza pandemics and the current COVID-19 pandemic, bacterial endotracheal superinfections are a well-known risk factor for higher morbidity and mortality. The goal of this study was to investigate the influence of a structured, objective, microbiological monitoring program on the prognosis of COVID-19 patients with mechanical ventilation.
Methods: A structured microbiological monitoring program (at intubation, then every 3 days) included collection of endotracheal material. Data analysis focused on the spectrum of bacterial pathogens, mortality, as well as intensive care unit (ICU), hospital, and mechanical ventilation duration.
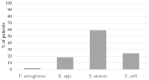
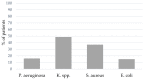
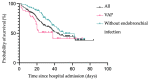
Results: A total of 29% of the patients showed bacterial coinfection at the time of intubation, and within 48 h, 56% developed ventilator-associated pneumonia (VAP). Even though patients with VAP had significantly longer ICU, hospital, and mechanical ventilation durations, there was no significant difference in mortality between patients with VAP pneumonia and patients without bacterial infection.
Conclusion: VAP is a common complication in COVID-19 patients. In contrast to already published studies, in our study implementing a structured microbiological monitoring program, COVID-19 patients with bacterial coinfection or VAP did not show higher mortality. Thus, a standardized, objective, microbiological screening can help detect coinfection and ventilator-associated infections, refining anti-infective therapy and positively influencing patient outcomes.
Keywords: COVID-19; ICU; SARS-CoV-2; bacterial spectrum; ventilator-associated pneumonia.
Conflict of interest statement
Tobias Lahmer reports travel grants and speaking fees from Gilead, Pfizer, MSD, Cytosorbents, Fresenius, and ADVITOS unrelated to this research. All other authors have no interests to declare. The other authors declare no competing interests.
Figures

References
-
- Warren D.K., Shukla S.J., Olsen M.A., Kollef M.H., Hollenbeak C.S., Cox M.J., Cohen M.M., Fraser V.J. Outcome and Attributable Cost of Ventilator-Associated Pneumonia among Intensive Care Unit Patients in a Suburban Medical Center. Crit. Care Med. 2003;31:1312–1317. doi: 10.1097/01.CCM.0000063087.93157.06. - DOI - PubMed
-
- Torres A., Niederman M.S., Chastre J., Ewig S., Fernandez-Vandellos P., Hanberger H., Kollef M., Bassi G.L., Luna C.M., Martin-Loeches I., et al. International ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Eur. Respir. J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. - DOI - PubMed
-
- Pickens C.O., Gao C.A., Cuttica M.J., Smith S.B., Pesce L.L., Grant R.A., Kang M., Morales-Nebreda L., Bavishi A.A., Arnold J.M., et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. Am. J. Respir. Crit. Care Med. 2021;204:921–932. doi: 10.1164/rccm.202106-1354OC. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

