The Rationale of Complement Blockade of the MCPggaac Haplotype following Atypical Hemolytic Uremic Syndrome of Three Southeastern European Countries with a Literature Review
- PMID: 37685848
- PMCID: PMC10487996
- DOI: 10.3390/ijms241713041
The Rationale of Complement Blockade of the MCPggaac Haplotype following Atypical Hemolytic Uremic Syndrome of Three Southeastern European Countries with a Literature Review
Abstract
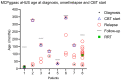
We present eight cases of the homozygous MCPggaac haplotype, which is considered to increase the likelihood and severity of atypical hemolytic uremic syndrome (aHUS), especially in combination with additional risk aHUS mutations. Complement blockade (CBT) was applied at a median age of 92 months (IQR 36-252 months). The median number of relapses before CBT initiation (Eculizumab) was two. Relapses occurred within an average of 22.16 months (median 17.5, minimum 8 months, and maximum 48 months) from the first subsequent onset of the disease (6/8 patients). All cases were treated with PI/PEX, and rarely with renal replacement therapy (RRT). When complement blockade was applied, children had no further disease relapses. Children with MCPggaac haplotype with/without additional gene mutations can achieve remission through renal replacement therapy without an immediate need for complement blockade. If relapse of aHUS occurs soon after disease onset or relapses are repeated frequently, a permanent complement blockade is required. However, the duration of such a blockade remains uncertain. If complement inhibition is not applied within 4-5 relapses, proteinuria and chronic renal failure will eventually occur.
Keywords: MCPggaac; Southeastern Europe; aHUS; children; complement blockade.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Esparza-Gordillo J., Jorge E., Garrido C.A., Carreras L., López-Trascasa M., Sánchez-Corral P., Decordoba S. Insights into hemolytic uremic syndrome: Segregation of three independent predisposition factors in a large, multiple affected pedigree. Mol. Immunol. 2006;43:1769–1775. doi: 10.1016/j.molimm.2005.11.008. - DOI - PubMed
-
- Esparza-Gordillo J., Goicoechea de Jorge E., Buil A., Carreras Berges L., López-Trascasa M., Sánchez-Corral P., de Córdoba S.R. Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum. Mol. Genet. 2005;14:703–712. doi: 10.1093/hmg/ddi066. - DOI - PubMed
-
- Le Clech A., Simon-Tillaux N., Provôt F., Delmas Y., Vieira-Martins P., Limou S., Halimi J.-M., Le Quintrec M., Lebourg L., Grangé S., et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 2019;95:1443–1452. doi: 10.1016/j.kint.2019.01.023. - DOI - PubMed
-
- Fidalgo T., Martinho P., Pinto C.S., Oliveira A.C., Salvado R., Borràs N., Coucelo M., Manco L., Maia T., Mendes M.J., et al. Combined study of ADAMTS13 and complement genes in the diagnosis of thrombotic microangiopathies using next-generation sequencing. Res. Pract. Thromb. Haemost. 2017;1:69–80. doi: 10.1002/rth2.12016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous