Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation
- PMID: 37686024
- PMCID: PMC10487653
- DOI: 10.3390/ijms241713219
Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation
Abstract
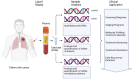
The detection of circulating tumor DNA (ctDNA) in liquid biopsy samples as an oncological marker is being used in clinical trials at every step of clinical management. As ctDNA-based liquid biopsy kits are developed and used in clinics, companies work towards increased convenience, accuracy, and cost over solid biopsies and other oncological markers. The technology used to differentiate ctDNA and cell-free DNA (cfDNA) continues to improve with new tests and methodologies being able to detect down to mutant allele frequencies of 0.001% or 1/100,000 copies. Recognizing this development in technology, the FDA has recently given pre-market approval and breakthrough device designations to multiple companies. The purpose of this review is to look at the utility of measuring total cfDNA, techniques used to differentiate ctDNA from cfDNA, and the utility of different ctDNA-based liquid biopsy kits using relevant articles from PubMed, clinicaltrials.gov, FDA approvals, and company newsletters. Measuring total cfDNA could be a cost-effective, viable prognostic marker, but various factors do not favor it as a monitoring tool during chemotherapy. While there may be a place in the clinic for measuring total cfDNA in the future, the lack of standardization means that it is difficult to move forward with large-scale clinical validation studies currently. While the detection of ctDNA has promising standardized liquid biopsy kits from various companies with large clinical trials ongoing, their applications in screening and minimal residual disease can suffer from lower sensitivity. However, researchers are working towards solutions to these issues with innovations in technology, multi-omics, and sampling. With great promise, further research is needed before liquid biopsies can be recommended for everyday clinical management.
Keywords: cfDNA; ctDNA; liquid biopsy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cancer World Health Organization. [(accessed on 15 March 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
-
- Incidence and Relative Survival by Stage at Diagnosis for Common Cancers Centers for Disease Control and Prevention. Published 10 November 2021. [(accessed on 15 March 2023)]; Available online: https://www.cdc.gov/cancer/uscs/about/data-briefs/no25-incidence-relativ....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

