Structures of the Insecticidal Toxin Complex Subunit XptA2 Highlight Roles for Flexible Domains
- PMID: 37686027
- PMCID: PMC10487846
- DOI: 10.3390/ijms241713221
Structures of the Insecticidal Toxin Complex Subunit XptA2 Highlight Roles for Flexible Domains
Abstract
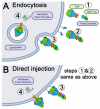
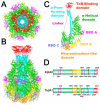
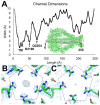
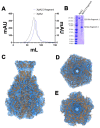
The Toxin Complex (Tc) superfamily consists of toxin translocases that contribute to the targeting, delivery, and cytotoxicity of certain pathogenic Gram-negative bacteria. Membrane receptor targeting is driven by the A-subunit (TcA), which comprises IgG-like receptor binding domains (RBDs) at the surface. To better understand XptA2, an insect specific TcA secreted by the symbiont X. nematophilus from the intestine of entomopathogenic nematodes, we determined structures by X-ray crystallography and cryo-EM. Contrary to a previous report, XptA2 is pentameric. RBD-B exhibits an indentation from crystal packing that indicates loose association with the shell and a hotspot for possible receptor binding or a trigger for conformational dynamics. A two-fragment XptA2 lacking an intact linker achieved the folded pre-pore state like wild type (wt), revealing no requirement of the linker for protein folding. The linker is disordered in all structures, and we propose it plays a role in dynamics downstream of the initial pre-pore state.
Keywords: Cryo-EM; TcA; X-ray crystallography; toxin translocase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chaston J.M., Suen G., Tucker S.L., Andersen A.W., Bhasin A., Bode E., Bode H.B., Brachmann A.O., Cowles C.E., Cowles K.N., et al. The entomopathogenic bacterial endosymbionts Xenorhabdus and Photorhabdus: Convergent lifestyles from divergent genomes. PLoS ONE. 2011;6:e27909. doi: 10.1371/journal.pone.0027909. - DOI - PMC - PubMed
-
- Blackburn M.B., Domek J.M., Gelman D.B., Hu J.S. The broadly insecticidal Photorhabdus luminescens toxin complex a (Tca): Activity against the Colorado potato beetle, Leptinotarsa decemlineata, and sweet potato whitefly, Bemisia tabaci. J Insect Sci. 2005;5:32. doi: 10.1093/jis/5.1.32. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

