Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline
- PMID: 37686140
- PMCID: PMC10487936
- DOI: 10.3390/ijms241713334
Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline
Abstract
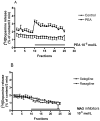
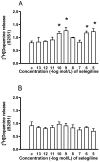
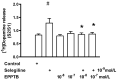
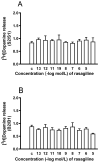
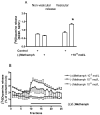
Selegiline and rasagiline are two selective monoamine oxidase B (MAO-B) inhibitors used in the treatment of Parkinson's disease. In their clinical application, however, differences in L-dopa-sparing potencies have been observed. The aim of this study was to find neurochemical and behavioral explanations for the antiparkinsonian effects of these drugs. We found that selegiline possesses a dopaminergic enhancer effect: it stimulated the electrically induced [3H]dopamine release without influencing the resting [3H]dopamine release from rat striatal slices in 10-10-10-9 mol/L concentrations. Rasagiline added in 10-13 to 10-5 mol/L concentrations did not alter the resting or electrically stimulated [3H]dopamine release. Rasagiline (10-9 mol/L), however, suspended the stimulatory effect of selegiline on the electrically induced [3H]dopamine release. The trace amine-associated receptor 1 (TAAR1) antagonist EPPTB (10-8-10-7 mol/L) also inhibited the stimulatory effect of selegiline on [3H]dopamine release. The effect of selegiline in its enhancer dose (5.33 nmol/kg) against tetrabenazine-induced learning deficit measured in a shuttle box apparatus was abolished by a 5.84 nmol/kg dose of rasagiline. The selegiline metabolite (-)methamphetamine (10-9 mol/L) also exhibited enhancer activity on [3H]dopamine release. We have concluded that selegiline acts as an MAO-B inhibitor and a dopaminergic enhancer drug, and the latter relates to an agonist effect on TAAR1. In contrast, rasagiline is devoid of enhancer activity but may act as an antagonist on TAAR1.
Keywords: [3H]dopamine release; conditioned avoidance response; dopaminergic activity enhancer effect; monoamine oxidase inhibition; rasagiline; rat striatum; selegiline; trace amine-associated receptor 1.
Conflict of interest statement
We declare this research work was partly supported by the Fujimoto Pharmaceutical Corporation, Osaka, Japan.
Figures





Similar articles
-
Monoamine oxidase-inhibition and MPTP-induced neurotoxicity in the non-human primate: comparison of rasagiline (TVP 1012) with selegiline.J Neural Transm (Vienna). 2001;108(8-9):985-1009. doi: 10.1007/s007020170018. J Neural Transm (Vienna). 2001. PMID: 11716151
-
Rat striatal monoamine oxidase-B inhibition by l-deprenyl and rasagiline: its relationship to 2-phenylethylamine-induced stereotypy and Parkinson's disease.Parkinsonism Relat Disord. 2002 Mar;8(4):247-53. doi: 10.1016/s1353-8020(01)00011-6. Parkinsonism Relat Disord. 2002. PMID: 12039419
-
Selegiline ameliorates depression-like behaviors in rodents and modulates hippocampal dopaminergic transmission and synaptic plasticity.Behav Brain Res. 2019 Feb 1;359:353-361. doi: 10.1016/j.bbr.2018.10.032. Epub 2018 Oct 22. Behav Brain Res. 2019. PMID: 30359642
-
Comprehensive review of rasagiline, a second-generation monoamine oxidase inhibitor, for the treatment of Parkinson's disease.Clin Ther. 2007 Sep;29(9):1825-49. doi: 10.1016/j.clinthera.2007.09.021. Clin Ther. 2007. PMID: 18035186 Review.
-
Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline, in Parkinson's disease.J Neural Transm (Vienna). 2018 Nov;125(11):1735-1749. doi: 10.1007/s00702-018-1853-9. Epub 2018 Feb 7. J Neural Transm (Vienna). 2018. PMID: 29417334 Review.
Cited by
-
Regulation by Trace Amine-Associated Receptor 1 (TAAR1) of Dopaminergic-GABAergic Interaction in the Striatum: Effects of the Enhancer Drug (-)BPAP.Neurochem Res. 2025 Feb 4;50(2):94. doi: 10.1007/s11064-025-04337-7. Neurochem Res. 2025. PMID: 39903411 Free PMC article.
-
Protective Effect of Selegiline (R-deprenyl) in Aminoglycoside-Induced Hearing Loss.Neurochem Res. 2025 Jun 13;50(3):200. doi: 10.1007/s11064-025-04446-3. Neurochem Res. 2025. PMID: 40512245 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

