Melatonin Prevents Depression but Not Anxiety-like Behavior Produced by the Chemotherapeutic Agent Temozolomide: Implication of Doublecortin Cells and Hilar Oligodendrocytes
- PMID: 37686181
- PMCID: PMC10487426
- DOI: 10.3390/ijms241713376
Melatonin Prevents Depression but Not Anxiety-like Behavior Produced by the Chemotherapeutic Agent Temozolomide: Implication of Doublecortin Cells and Hilar Oligodendrocytes
Abstract
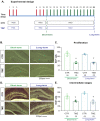
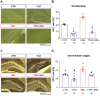
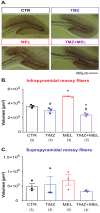
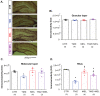
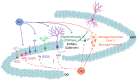
Melatonin is a hormone synthesized by the pineal gland with neuroprotective and neurodevelopmental effects. Also, melatonin acts as an antidepressant by modulating the generation of new neurons in the dentate gyrus of the hippocampus. The positive effects of melatonin on behavior and neural development may suggest it is used for reverting stress but also for the alterations produced by chemotherapeutic drugs influencing behavior and brain plasticity. In this sense, temozolomide, an alkylating/anti-proliferating agent used in treating brain cancer, is associated with decreased cognitive functions and depression. We hypothesized that melatonin might prevent the effects of temozolomide on depression- and anxiety-like behavior by modulating some aspects of the neurogenic process in adult Balb/C mice. Mice were treated with temozolomide (25 mg/kg) for three days of two weeks, followed by melatonin (8 mg/kg) for fourteen days. Temozolomide produced short- and long-term decrements in cell proliferation (Ki67-positive cells: 54.89% and 53.38%, respectively) and intermediate stages of the neurogenic process (doublecortin-positive cells: 68.23% and 50.08%, respectively). However, melatonin prevented the long-term effects of temozolomide with the increased number of doublecortin-positive cells (47.21%) and the immunoreactivity of 2' 3'-Cyclic-nucleotide-3 phosphodiesterase (CNPase: 82.66%), an enzyme expressed by mature oligodendrocytes, in the hilar portion of the dentate gyrus. The effects of melatonin in the temozolomide group occurred with decreased immobility in the forced swim test (45.55%) but not anxiety-like behavior. Thus, our results suggest that melatonin prevents the harmful effects of temozolomide by modulating doublecortin cells, hilar oligodendrocytes, and depression-like behavior tested in the forced swim test. Our study could point out melatonin's beneficial effects for counteracting temozolomide's side effects.
Keywords: adult neurogenesis; anxiety; depression; hippocampus; melatonin; oligodendrocytes; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Perry J.R., Bélanger K., Mason W.P., Fulton D., Kavan P., Easaw J., Shields C., Kirby S., Macdonald D.R., Eisenstat D.D., et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J. Clin. Oncol. 2010;28:2051–2057. doi: 10.1200/JCO.2009.26.5520. - DOI - PubMed
-
- Weller M., Tabatabai G., Kästner B., Felsberg J., Steinbach J.P., Wick A., Schnell O., Hau P., Herrlinger U., Sabel M.C., et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin. Cancer Res. 2015;21:2057–2064. doi: 10.1158/1078-0432.CCR-14-2737. - DOI - PubMed
-
- [(accessed on 3 July 2022)]. Available online: https://gco.iarc.fr/today/fact-sheets-cancers.
-
- [(accessed on 3 July 2022)]. Available online: https://www.cancer.net/cancer-types/brain-tumor/types-treatment.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

