Evaluation of Human Hepatocyte Drug Metabolism Carrying High-Risk or Protection-Associated Liver Disease Genetic Variants
- PMID: 37686209
- PMCID: PMC10487897
- DOI: 10.3390/ijms241713406
Evaluation of Human Hepatocyte Drug Metabolism Carrying High-Risk or Protection-Associated Liver Disease Genetic Variants
Abstract
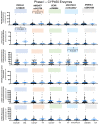
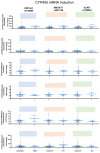
Metabolic-dysfunction-associated steatotic liver disease (MASLD), which affects 30 million people in the US and is anticipated to reach over 100 million by 2030, places a significant financial strain on the healthcare system. There is presently no FDA-approved treatment for MASLD despite its public health significance and financial burden. Understanding the connection between point mutations, liver enzymes, and MASLD is important for comprehending drug toxicity in healthy or diseased individuals. Multiple genetic variations have been linked to MASLD susceptibility through genome-wide association studies (GWAS), either increasing MASLD risk or protecting against it, such as PNPLA3 rs738409, MBOAT7 rs641738, GCKR rs780094, HSD17B13 rs72613567, and MTARC1 rs2642438. As the impact of genetic variants on the levels of drug-metabolizing cytochrome P450 (CYP) enzymes in human hepatocytes has not been thoroughly investigated, this study aims to describe the analysis of metabolic functions for selected phase I and phase II liver enzymes in human hepatocytes. For this purpose, fresh isolated primary hepatocytes were obtained from healthy liver donors (n = 126), and liquid chromatography-mass spectrometry (LC-MS) was performed. For the cohorts, participants were classified into minor homozygotes and nonminor homozygotes (major homozygotes + heterozygotes) for five gene polymorphisms. For phase I liver enzymes, we found a significant difference in the activity of CYP1A2 in human hepatocytes carrying MBOAT7 (p = 0.011) and of CYP2C8 in human hepatocytes carrying PNPLA3 (p = 0.004). It was also observed that the activity of CYP2C9 was significantly lower in human hepatocytes carrying HSD17B13 (p = 0.001) minor homozygous compared to nonminor homozygous. No significant difference in activity of CYP2E1, CYP2C8, CYP2D6, CYP2E1, CYP3A4, ECOD, FMO, MAO, AO, and CES2 and in any of the phase II liver enzymes between human hepatocytes carrying genetic variants for PNPLA3 rs738409, MBOAT7 rs641738, GCKR rs780094, HSD17B13 rs72613567, and MTARC1 rs2642438 were observed. These findings offer a preliminary assessment of the influence of genetic variations on drug-metabolizing cytochrome P450 (CYP) enzymes in healthy human hepatocytes, which may be useful for future drug discovery investigations.
Keywords: end-stage liver disease; genetic polymorphisms; metabolic dysfunction-associated steatohepatitis; metabolic-dysfunction-associated steatotic liver disease; nonalcoholic steatohepatitis.
Conflict of interest statement
A.S.-G. is an inventor on a patent application that describes the use of transcription factors to treat chronic liver failure (US20140249209). E.N.T. and A.S.-G. are inventors on a provisional patent application related to methods to enhance hepatic functions in failing human livers (PCT/US2020/055500). A.S.-G. is a cofounder and has a financial interest in Von Baer Wolff Inc., a company focused on biofabrication of autologous human hepatocytes from stem cell technology. A.S.-G. and A.O. are cofounders and have a financial interest in Pittsburgh ReLiver Inc., a company focused on reprogramming hepatocytes in liver failure. All interests are managed by the Conflict of Interest Office at the University of Pittsburgh in accordance with their policies.
Figures



References
-
- Jain M.R., Giri S.R., Trivedi C., Bhoi B., Rath A., Vanage G., Vyas P., Ranvir R., Patel P.R. Saroglitazar, a novel PPARalpha/gamma agonist with predominant PPARalpha activity, shows lipid-lowering and insulin-sensitizing effects in preclinical models. Pharmacol. Res. Perspect. 2015;3:e00136. doi: 10.1002/prp2.136. - DOI - PMC - PubMed
-
- Speliotes E.K., Yerges-Armstrong L.M., Wu J., Hernaez R., Kim L.J., Palmer C.D., Gudnason V., Eiriksdottir G., Garcia M.E., Launer L.J., et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;3:e1001324. doi: 10.1371/journal.pgen.1001324. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

