ERK1/2-Dependent Phosphorylation of GABAB1(S867/T872), Controlled by CaMKIIβ, Is Required for GABAB Receptor Degradation under Physiological and Pathological Conditions
- PMID: 37686242
- PMCID: PMC10488028
- DOI: 10.3390/ijms241713436
ERK1/2-Dependent Phosphorylation of GABAB1(S867/T872), Controlled by CaMKIIβ, Is Required for GABAB Receptor Degradation under Physiological and Pathological Conditions
Abstract
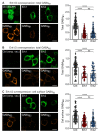
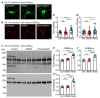
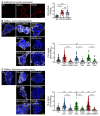
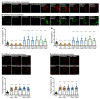
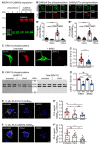
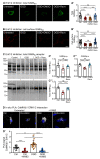
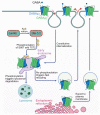
GABAB receptor-mediated inhibition is indispensable for maintaining a healthy neuronal excitation/inhibition balance. Many neurological diseases are associated with a disturbed excitation/inhibition balance and downregulation of GABAB receptors due to enhanced sorting of the receptors to lysosomal degradation. A key event triggering the downregulation of the receptors is the phosphorylation of S867 in the GABAB1 subunit mediated by CaMKIIβ. Interestingly, close to S867 in GABAB1 exists another phosphorylation site, T872. Therefore, the question arose as to whether phosphorylation of T872 is involved in downregulating the receptors and whether phosphorylation of this site is also mediated by CaMKIIβ or by another protein kinase. Here, we show that mutational inactivation of T872 in GABAB1 prevented the degradation of the receptors in cultured neurons. We found that, in addition to CaMKIIβ, also ERK1/2 is involved in the degradation pathway of GABAB receptors under physiological and ischemic conditions. In contrast to our previous view, CaMKIIβ does not appear to directly phosphorylate S867. Instead, the data support a mechanism in which CaMKIIβ activates ERK1/2, which then phosphorylates S867 and T872 in GABAB1. Blocking ERK activity after subjecting neurons to ischemic stress completely restored downregulated GABAB receptor expression to normal levels. Thus, preventing ERK1/2-mediated phosphorylation of S867/T872 in GABAB1 is an opportunity to inhibit the pathological downregulation of the receptors after ischemic stress and is expected to restore a healthy neuronal excitation/inhibition balance.
Keywords: CaMKII; ERK1/2; GABAB receptors; cerebral ischemia; degradation; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) β-Dependent Phosphorylation of GABAB1 Triggers Lysosomal Degradation of GABAB Receptors via Mind Bomb-2 (MIB2)-Mediated Lys-63-Linked Ubiquitination.Mol Neurobiol. 2019 Feb;56(2):1293-1309. doi: 10.1007/s12035-018-1142-5. Epub 2018 Jun 7. Mol Neurobiol. 2019. PMID: 29881949 Free PMC article.
-
NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1.Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13924-9. doi: 10.1073/pnas.1000909107. Epub 2010 Jul 19. Proc Natl Acad Sci U S A. 2010. PMID: 20643921 Free PMC article.
-
Lys-63-linked Ubiquitination of γ-Aminobutyric Acid (GABA), Type B1, at Multiple Sites by the E3 Ligase Mind Bomb-2 Targets GABAB Receptors to Lysosomal Degradation.J Biol Chem. 2016 Oct 7;291(41):21682-21693. doi: 10.1074/jbc.M116.750968. Epub 2016 Aug 29. J Biol Chem. 2016. PMID: 27573246 Free PMC article.
-
Clinical potential of GABAB receptor modulators.CNS Drug Rev. 2005 Autumn;11(3):317-34. doi: 10.1111/j.1527-3458.2005.tb00049.x. CNS Drug Rev. 2005. PMID: 16389296 Free PMC article. Review.
-
The role of GABAB receptors in the subcortical pathways of the mammalian auditory system.Front Endocrinol (Lausanne). 2023 Aug 11;14:1195038. doi: 10.3389/fendo.2023.1195038. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37635966 Free PMC article. Review.
Cited by
-
The E3 ubiquitin ligase MARCH1 mediates downregulation of plasma membrane GABAB receptors under ischemic conditions by inhibiting fast receptor recycling.Sci Rep. 2025 Jan 8;15(1):1330. doi: 10.1038/s41598-025-85842-1. Sci Rep. 2025. PMID: 39779794 Free PMC article.
-
Restoring GABAB receptor expression in the ventral tegmental area of methamphetamine addicted mice inhibits locomotor sensitization and drug seeking behavior.Front Mol Neurosci. 2024 Feb 7;17:1347228. doi: 10.3389/fnmol.2024.1347228. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38384279 Free PMC article.
-
Neurotransmitters: an emerging target for therapeutic resistance to tumor immune checkpoint inhibitors.Mol Cancer. 2025 Aug 11;24(1):216. doi: 10.1186/s12943-025-02413-8. Mol Cancer. 2025. PMID: 40790592 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

